Membrane protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane (integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane.
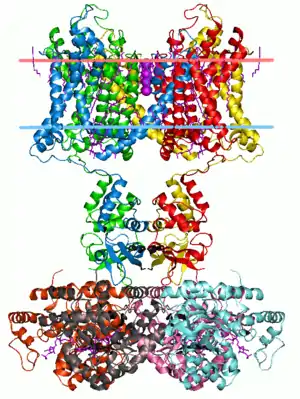
Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs.[1] Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment.
Function
Membrane proteins perform a variety of functions vital to the survival of organisms:[2]
- Membrane receptor proteins relay signals between the cell's internal and external environments.
- Transport proteins move molecules and ions across the membrane. They can be categorized according to the Transporter Classification database.
- Membrane enzymes may have many activities, such as oxidoreductase, transferase or hydrolase.[3]
- Cell adhesion molecules allow cells to identify each other and interact. For example, proteins involved in immune response
The localization of proteins in membranes can be predicted reliably using hydrophobicity analyses of protein sequences, i.e. the localization of hydrophobic amino acid sequences.
Integral membrane proteins
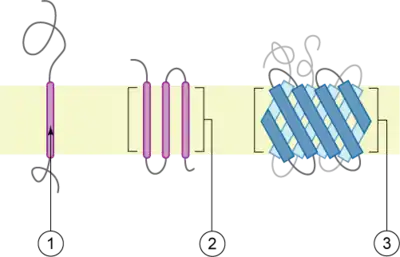
The membrane is represented in light-brown.
Integral membrane proteins are permanently attached to the membrane. Such proteins can be separated from the biological membranes only using detergents, nonpolar solvents, or sometimes denaturing agents. They can be classified according to their relationship with the bilayer:
- Integral polytopic proteins are transmembrane proteins that span across the membrane more than once. These proteins may have different transmembrane topology.[4][5] These proteins have one of two structural architectures:
- Helix bundle proteins, which are present in all types of biological membranes;
- Beta barrel proteins, which are found only in outer membranes of Gram-negative bacteria, and outer membranes of mitochondria and chloroplasts.[6]
- Bitopic proteins are transmembrane proteins that span across the membrane only once. Transmembrane helices from these proteins have significantly different amino acid distributions to transmembrane helices from polytopic proteins.[7]
- Integral monotopic proteins are integral membrane proteins that are attached to only one side of the membrane and do not span the whole way across.
Peripheral membrane proteins
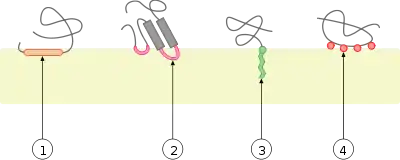
Peripheral membrane proteins are temporarily attached either to the lipid bilayer or to integral proteins by a combination of hydrophobic, electrostatic, and other non-covalent interactions. Peripheral proteins dissociate following treatment with a polar reagent, such as a solution with an elevated pH or high salt concentrations.
Integral and peripheral proteins may be post-translationally modified, with added fatty acid, diacylglycerol[8] or prenyl chains, or GPI (glycosylphosphatidylinositol), which may be anchored in the lipid bilayer.
Polypeptide toxins
Polypeptide toxins and many antibacterial peptides, such as colicins or hemolysins, and certain proteins involved in apoptosis, are sometimes considered a separate category. These proteins are water-soluble but can aggregate and associate irreversibly with the lipid bilayer and become reversibly or irreversibly membrane-associated.
In genomes
Membrane proteins, like soluble globular proteins, fibrous proteins, and disordered proteins, are common.[9] It is estimated that 20–30% of all genes in most genomes encode for membrane proteins.[10][11] For instance, about 1000 of the ~4200 proteins of E. coli are thought to be membrane proteins, 600 of which have been experimentally verified to be membrane resident.[12] In humans, current thinking suggests that fully 30% of the genome encodes membrane proteins.[13]
In disease
Membrane proteins are the targets of over 50% of all modern medicinal drugs.[1] Among the human diseases in which membrane proteins have been implicated are heart disease, Alzheimer's and cystic fibrosis.[13]
Purification of membrane proteins
Although membrane proteins play an important role in all organisms, their purification has historically, and continues to be, a huge challenge for protein scientists. In 2008, 150 unique structures of membrane proteins were available,[14] and by 2019 only 50 human membrane proteins had had their structures elucidated.[13] In contrast, approximately 25% of all proteins are membrane proteins.[15] Their hydrophobic surfaces make structural and especially functional characterization difficult.[13][16] Detergents can be used to render membrane proteins water-soluble, but these can also alter protein structure and function.[13] Making membrane proteins water-soluble can also be achieved through engineering the protein sequence, replacing selected hydrophobic amino acids with hydrophilic ones, taking great care to maintain secondary structure while revising overall charge.[13]
Affinity chromatography is one of the best solutions for purification of membrane proteins. The activity of membrane proteins decreases very fast in contrast to other proteins. So, affinity chromatography provides a fast and specific purification of membrane proteins. The polyhistidine-tag is a commonly used tag for membrane protein purification,[17] and the alternative rho1D4 tag has also been successfully used.[18][19]
Further reading
- Johnson JE, Cornell RB (1999). "Amphitropic proteins: regulation by reversible membrane interactions (review)". Molecular Membrane Biology. 16 (3): 217–35. doi:10.1080/096876899294544. PMID 10503244.
- Alenghat FJ, Golan DE (2013). "Membrane protein dynamics and functional implications in mammalian cells". Current Topics in Membranes. 72: 89–120. doi:10.1016/b978-0-12-417027-8.00003-9. ISBN 9780124170278. PMC 4193470. PMID 24210428.
See also
- Annular lipid shell
- Carrier protein
- Inner nuclear membrane proteins
- Ion channel
- Ion pump (biology)
- List of MeSH codes (D12.776)
- Receptor (biochemistry)
- TMPad (TransMembrane Protein Helix-Packing Database)
- Transmembrane proteins
References
- Overington JP, Al-Lazikani B, Hopkins AL (December 2006). "How many drug targets are there?". Nature Reviews. Drug Discovery (Opinion). 5 (12): 993–6. doi:10.1038/nrd2199. PMID 17139284. S2CID 11979420.
- Almén MS, Nordström KJ, Fredriksson R, Schiöth HB (August 2009). "Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin". BMC Biology. 7: 50. doi:10.1186/1741-7007-7-50. PMC 2739160. PMID 19678920.
- Lin Y, Fuerst O, Granell M, Leblanc G, Lórenz-Fonfría V, Padrós E (August 2013). "The substitution of Arg149 with Cys fixes the melibiose transporter in an inward-open conformation". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1828 (8): 1690–9. doi:10.1016/j.bbamem.2013.03.003. PMID 23500619 – via Elsevier Science Direct.

- von Heijne G (December 2006). "Membrane-protein topology". Nature Reviews. Molecular Cell Biology. 7 (12): 909–18. doi:10.1038/nrm2063. PMID 17139331. S2CID 22218266.
- Gerald Karp (2009). Cell and Molecular Biology: Concepts and Experiments. John Wiley and Sons. pp. 128–. ISBN 978-0-470-48337-4. Retrieved 13 November 2010 – via Google Books.
- Selkrig J, Leyton DL, Webb CT, Lithgow T (August 2014). "Assembly of β-barrel proteins into bacterial outer membranes". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1843 (8): 1542–50. doi:10.1016/j.bbamcr.2013.10.009. PMID 24135059 – via Elsevier Science Direct.
- Baker JA, Wong WC, Eisenhaber B, Warwicker J, Eisenhaber F (July 2017). "Charged residues next to transmembrane regions revisited: "Positive-inside rule" is complemented by the "negative inside depletion/outside enrichment rule"". BMC Biology. 15 (1): 66. doi:10.1186/s12915-017-0404-4. PMC 5525207. PMID 28738801.

- Sun C, Benlekbir S, Venkatakrishnan P, Wang Y, Hong S, Hosler J, Tajkhorshid E, Rubinstein JL, Gennis RB (May 2018). "Structure of the alternative complex III in a supercomplex with cytochrome oxidase". Nature. 557 (7703): 123–126. Bibcode:2018Natur.557..123S. doi:10.1038/s41586-018-0061-y. PMC 6004266. PMID 29695868.
- Andreeva A, Howorth D, Chothia C, Kulesha E, Murzin AG (January 2014). "SCOP2 prototype: a new approach to protein structure mining". Nucleic Acids Research. 42 (Database issue): D310-4. doi:10.1093/nar/gkt1242. PMC 3964979. PMID 24293656.
- Liszewski K (1 October 2015). "Dissecting the Structure of Membrane Proteins". Genetic Engineering & Biotechnology News (paper). 35 (17): 1, 14, 16–17. doi:10.1089/gen.35.17.02.
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL (January 2001). "Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes" (PDF). Journal of Molecular Biology. 305 (3): 567–80. doi:10.1006/jmbi.2000.4315. PMID 11152613. S2CID 15769874. Archived from the original (PDF) on 2020-08-04 – via Semantic Scholar.

- Daley DO, Rapp M, Granseth E, Melén K, Drew D, von Heijne G (May 2005). "Global topology analysis of the Escherichia coli inner membrane proteome" (PDF). Science (Report). 308 (5726): 1321–3. Bibcode:2005Sci...308.1321D. doi:10.1126/science.1109730. PMID 15919996. S2CID 6942424 – via Semantic Scholar.

- Martin, Joseph; Sawyer, Abigail (2019). "Elucidating the Structure of Membrane Proteins". Tech News. BioTechniques (Print issue). Future Science. 66 (4): 167–170. doi:10.2144/btn-2019-0030. PMID 30987442.

- Carpenter EP, Beis K, Cameron AD, Iwata S (October 2008). "Overcoming the challenges of membrane protein crystallography". Current Opinion in Structural Biology. 18 (5): 581–6. doi:10.1016/j.sbi.2008.07.001. PMC 2580798. PMID 18674618.
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL (January 2001). "Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes" (PDF). Journal of Molecular Biology. 305 (3): 567–80. doi:10.1006/jmbi.2000.4315. PMID 11152613. S2CID 15769874. Archived from the original (PDF) on 2020-08-04 – via Semantic Scholar.

- Rawlings AE (June 2016). "Membrane proteins: always an insoluble problem?". Biochemical Society Transactions. 44 (3): 790–5. doi:10.1042/BST20160025. PMC 4900757. PMID 27284043.
- Hochuli E, Bannwarth W, Döbeli H, Gentz R, Stüber D (November 1988). "Genetic Approach to Facilitate Purification of Recombinant Proteins with a Novel Metal Chelate Adsorbent". Nature Biotechnology. 6 (11): 1321–1325. doi:10.1038/nbt1188-1321. S2CID 9518666.
- Locatelli-Hoops SC, Gorshkova I, Gawrisch K, Yeliseev AA (October 2013). "Expression, surface immobilization, and characterization of functional recombinant cannabinoid receptor CB2". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1834 (10): 2045–56. doi:10.1016/j.bbapap.2013.06.003. PMC 3779079. PMID 23777860.
- Cook BL, Steuerwald D, Kaiser L, Graveland-Bikker J, Vanberghem M, Berke AP, Herlihy K, Pick H, Vogel H, Zhang S (July 2009). "Large-scale production and study of a synthetic G protein-coupled receptor: human olfactory receptor 17-4". Proceedings of the National Academy of Sciences of the United States of America. 106 (29): 11925–30. Bibcode:2009PNAS..10611925C. doi:10.1073/pnas.0811089106. PMC 2715541. PMID 19581598.
External links
Organizations
- Membrane Protein Structural Dynamics Consortium
- Experts for Membrane Protein Research and Purification
Membrane protein databases
- TCDB - Transporter Classification database, a comprehensive classification of transmembrane transporter proteins
- Orientations of Proteins in Membranes (OPM) database - 3D structures of integral and peripheral membrane proteins arranged in the lipid bilayer
- Protein Data Bank of Transmembrane Proteins - 3D models of transmembrane proteins approximately arranged in the lipid bilayer.
- TransportDB - Genomics-oriented database of transporters from TIGR
- Membrane PDB - Database of 3D structures of integral membrane proteins and hydrophobic peptides with an emphasis on crystallization conditions
- Mpstruc database Archived 2013-12-25 at the Wayback Machine - A curated list of selected transmembrane proteins from the Protein Data Bank
- MemProtMD - a database of membrane protein structures simulated by coarse-grained molecular dynamics
- Membranome database provides information about bitopic proteins from several model organisms