Ocean acidification
Ocean acidification is the reduction in the pH of the Earth’s ocean. This process takes place over periods lasting decades or more. Its main cause is the absorption of carbon dioxide (CO2) from the atmosphere. This, in turn, increases CO2 concentrations in the ocean. Between 23 and 30% of the CO2 that is in the atmosphere dissolves into oceans, rivers and lakes.[1][2][3] Acidification is one of several effects of rising CO2 on the ocean. Other chemical changes to the ocean can also cause acidification.[4] As the ocean absorbs CO2, seawater chemistry changes, which changes the living conditions of marine species. Many different species are affected, especially organisms that rely on calcium carbonate shells and skeletons, like mollusks, oysters and corals. Organisms like these struggle to build those parts of their anatomy when ocean waters have increased acidity.[5] The main solution to present-day ocean acidification lies in reducing atmospheric CO2 levels through climate change mitigation.

2 levels between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas
When carbon dioxide is absorbed by the ocean, carbonic acid forms and quickly dissociates into a bicarbonate ion (HCO3⁻) and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the ocean pH of the ocean, causing acidification (this does not mean that seawater is acidic yet: it is still alkaline with a pH higher than 8). The lowered pH causes a decrease in the concentration of carbonate ions, which are the main building block for calcium carbonate (CaCO3) shells and skeletons. It also lowers the carbonate mineral saturation state. Ocean alkalinity is not changed by ocean acidification, but over long time periods alkalinity may increase due to carbonate dissolution and reduced formation of calcium carbonate shells.[6][7]
Between 1751 and 2021, the pH value of the ocean surface is estimated to have decreased from approximately 8.25 to 8.14.[1] This represents an increase of almost 30% in hydrogen ion concentration in the world's oceans (the pH scale is logarithmic, so a change of one in pH unit is equivalent to a tenfold change in hydrogen ion concentration).[8] Sea-surface pH and carbonate saturation states can vary depending on ocean depth and location. Colder and higher latitude waters have the capacity to absorb more CO2. This can increase acidification, lowering the pH and carbonate saturation states in these regions. Other factors that affect the atmosphere-ocean CO2 exchange, and therefore impact local ocean acidification, include: ocean currents (upwelling zones), proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel burning and agriculture.[9][10][11]
Decreased ocean pH has a range of potentially harmful effects for marine organisms. These include reduced calcification, depressed metabolic rates, lowered immune responses, and reduced energy for basic functions such as reproduction.[12] So the effects of ocean acidification are impacting marine ecosystems that provide food, livelihoods, and other ecosystem services for a large portion of humanity. Some 1 billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by coral reefs. Ongoing acidification of the oceans may therefore threaten future food chains linked with the oceans. [6][13]
A statement on ocean acidification by over 100 science academies recommends that by 2050, global CO2 emissions be reduced by at least 50% compared to 1990 levels.[14] The United Nations Sustainable Development Goal 14 ("Life below Water") also has a target to "minimize and address the impacts of ocean acidification".[15]
Ocean acidification has occurred previously in Earth's history. The resulting ecological collapse in the oceans had long-lasting effects on the global carbon cycle and climate.
Causes and carbon cycle




2 cycle between the atmosphere and the ocean
Human activities such as the combustion of fossil fuels and land-use changes have led to a new flux of CO
2 into the atmosphere. About 45% has remained in the atmosphere, about 24% has been absorbed by the ocean,[17] and about 32% taken up by land (terrestrial plants).[18]
The carbon cycle describes the fluxes of carbon dioxide (CO
2) between the oceans, terrestrial biosphere, lithosphere,[19] and atmosphere. The carbon cycle involves both organic compounds such as cellulose and inorganic carbon compounds such as carbon dioxide, carbonate ion, and bicarbonate ion, together referenced as dissolved inorganic carbon (DIC). The inorganic compounds are particularly relevant when discussing ocean acidification for they include many forms of dissolved CO
2 present in the Earth's oceans.[20]
When CO
2 dissolves, it reacts with water to form a balance of ionic and non-ionic chemical species: dissolved free carbon dioxide (CO
2(aq)), carbonic acid (H
2CO
3), bicarbonate (HCO−
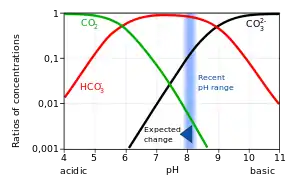
3) and carbonate (CO2−
3). The ratio of these species depends on factors such as seawater temperature, pressure and salinity (as shown in a Bjerrum plot). These different forms of dissolved inorganic carbon are transferred from an ocean's surface to its interior by the ocean's solubility pump.
The resistance of an area of ocean to absorbing atmospheric CO
2 is known as the Revelle factor.
Ocean acidification has been compared to anthropogenic climate change and called the "evil twin of global warming"and "the other CO2 problem".[21][22] Increased ocean temperatures and oxygen loss act concurrently with ocean acidification and constitute the "deadly trio" of climate change pressures on the marine environment.[23] Freshwater bodies also appear to be acidifying, although this is a more complex and less obvious phenomenon.[24][25]
An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes.[26][27]
Mechanism of acidification
Dissolving CO
2 in seawater increases the hydrogen ion (H+
) concentration in the ocean, and thus decreases ocean pH, as follows:[28]
Ocean surface hydrogen ion concentrations have increased by approximately 30% since the beginning of the industrial revolution. [29] Since the industrial revolution began, it is estimated that surface ocean pH has dropped by slightly more than 0.1 units on the logarithmic scale of pH. Under a “business as usual” model, where little effort is made to curb emissions, it is expected to drop by a further 0.16 to 0.44 pH unit [30](an additional two to four times of today's post-industrial H+ concentrations) by 2100, the impacts being most severe for coral reefs and other shelled marine organisms,[31] as well as the economies and people that depend on the ecosystem services they provide. Thus, the degree of change to ocean chemistry, including ocean pH, will depend on the mitigation and emissions pathways taken by governments and nations.[32]: 704
Although the largest changes are expected in the future,[33] a report from NOAA scientists found large quantities of water undersaturated in aragonite are already upwelling close to the Pacific continental shelf area of North America.[16] Continental shelves play an important role in marine ecosystems since most marine organisms live or are spawned there, and though the study only dealt with the area from Vancouver to Northern California, the authors suggest that other shelf areas may be experiencing similar effects.[34]
| Time | pH | pH change relative to pre-industrial |
Source | H+ concentration change relative to pre-industrial |
|---|---|---|---|---|
| Pre-industrial (18th century) | 8.179 | analysed field[36] | ||
| Recent past (1990s) | 8.104 | −0.075 | field[36] | + 18.9% |
| Present levels | ~8.069 | −0.11 | field[2][8][37][38] | + 28.8% |
| 2050 (2×CO 2 = 560 ppm) |
7.949 | −0.230 | model[35] | + 69.8% |
| 2100 (IS92a)[39] | 7.824 | −0.355 | model[35] | + 126.5% |

In shallow coastal and shelf regions, a number of factors interplay to affect air-ocean CO2 exchange and resulting pH change.[40][41] These include biological processes, such as photosynthesis and respiration,[42] as well as water upwelling.[43] Also, ecosystem metabolism in freshwater sources reaching coastal waters can lead to large, but local, pH changes,[40] with the rates of biologically induced pH change dependent on local water temperature, among other factors.
Observed rates
If we continue emitting CO2 at the same rate, by 2100 ocean acidity will increase by about 150 percent, a rate that has not been experienced for at least 400,000 years.
— UK Ocean Acidification Research Programme, 2015[44]
One of the first detailed datasets to examine how pH varied over 8 years at a specific north temperate coastal location found that acidification had strong links to in situ benthic species dynamics and that the variation in ocean pH may cause calcareous species to perform more poorly than noncalcareous species in years with low pH and predicts consequences for near-shore benthic ecosystems.[45][46]
| Part of a series on the |
| Carbon cycle |
|---|
 |
|
Current rates of ocean acidification have been compared with the greenhouse event at the Paleocene–Eocene boundary (about 56 million years ago) when surface ocean temperatures rose by 5–6 degrees Celsius. Surface ecosystems experienced stress, yet bottom-dwelling organisms in the deep ocean experienced a major extinction.[47] The rate of carbon addition to the atmosphere-ocean system at present day is about ten times the rate of carbon addition than at the Paleocene–Eocene boundary.[48] While the current ocean acidification is on a path to reach lower pH levels than any other level recorded in the last 300 million years,[49][50] the rate of carbon addition is unparalleled, therefore the current and projected acidification, namely the decrease in carbonate saturation states has been described as unprecedented in the geological record.[51] A National Research Council study released in April 2010 likewise concluded that "the level of acid in the oceans is increasing at an unprecedented rate".[52][53] A 2012 paper in the journal Science examined the geological record in an attempt to find a historical analog for current global conditions as well as those of the future. The researchers determined that the current rate of ocean acidification is faster than at any time in the past 300 million years.[54][55]
A review by climate scientists at the RealClimate blog, of a 2005 report by the Royal Society of the UK similarly highlighted the centrality of the rates of change in the present anthropogenic acidification process, writing:[56]
The natural pH of the ocean is determined by a need to balance the deposition and burial of CaCO3 on the sea floor against the influx of Ca2+ and CO2−3 into the ocean from dissolving rocks on land, called weathering. These processes stabilize the pH of the ocean, by a mechanism called CaCO3 compensation...The point of bringing it up again is to note that if the CO2 concentration of the atmosphere changes more slowly than this, as it always has throughout the Vostok record, the pH of the ocean will be relatively unaffected because CaCO3 compensation can keep up.
In the 15-year period 1995–2010 alone, acidity has increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.[57] According to a statement in July 2012 by Jane Lubchenco, head of the U.S. National Oceanic and Atmospheric Administration "surface waters are changing much more rapidly than initial calculations have suggested. It's yet another reason to be very seriously concerned about the amount of carbon dioxide that is in the atmosphere now and the additional amount we continue to put out."[58]
A 2013 study claimed acidity was increasing at a rate 10 times faster than in any of the evolutionary crises in Earth's history.[59] In a synthesis report published in Science in 2015, 22 leading marine scientists stated that CO2 from burning fossil fuels is changing the oceans' chemistry more rapidly than at any time since the Great Dying, Earth's most severe known extinction event, emphasizing that the 2 °C maximum temperature increase agreed upon by governments reflects too small a cut in emissions to prevent "dramatic impacts" on the world's oceans, with lead author Jean-Pierre Gattuso remarking that "The ocean has been minimally considered at previous climate negotiations. Our study provides compelling arguments for a radical change at the UN conference (in Paris) on climate change".[60]
The rate at which ocean acidification will occur may be influenced by the rate of surface ocean warming, because warm waters will not absorb as much CO2.[61] Therefore, greater seawater warming could limit CO2 absorption and lead to a smaller change in pH for a given increase in CO2.[61] The difference in changes in temperature between basins is one of the main reasons for the differences in acidification rates in different localities. At present, the surface ocean is acidifying at a rate of 0.003-0.026 units per decade. However this rate is faster in the polar regions (-0.002 to -0.026 per decade) than at the subtropical regions (-0.016 to -0.020 per decade)[62].: 83
| Location | Acidification rate (10−3 pH units / year) | Period | Data source |
|---|---|---|---|
| Iceland[63] | -2.4 | 1984 – 2009 | Direct measurements |
| Drake Passage[64] | -1.8 | 2002 – 2012 | Direct measurements |
| Canary (ESTOC)[65] | -1.7 | 1995 – 2004 | Direct measurements |
| Hawaii (HOT)[66] | -1.9 | 1989 – 2007 | Direct measurements |
| Bermuda (BATS)[67] | -1.7 | 1984 – 2012 | Direct measurements |
| Coral Sea[68] | -0.2 | ~1700 – ~1990 | Proxy reconstruction |
| Eastern Mediterranean[69] | -2.3 | 1964 – 2005 | Proxy reconstruction |
Predicted future rates
Earth System Models project that, by around 2008, ocean acidity exceeded historical analogues[70] and, in combination with other ocean biogeochemical changes, could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean beginning as early as 2100.[71]
If the 'business as usual' model for human activity persists, model projections estimate that surface ocean pH could decrease by 0.16 to 0.44 units compared to the present day by the end of the century [72]: 608 The ocean has not experienced this level of acidity for 300 million years.[73]
An ecological tipping point was projected to occur by 2030 and no later than 2038.[74] Thomas Lovejoy, former chief biodiversity advisor to the World Bank, has suggested that "the acidity of the oceans will more than double in the next 40 years. He says this rate is 100 times faster than any changes in ocean acidity in the last 20 million years, making it unlikely that marine life can somehow adapt to the changes."[75] It is predicted that, by the year 2100, If co-occurring biogeochemical changes influence the delivery of ocean goods and services, then they could also have a considerable effect on human welfare for those who rely heavily on the ocean for food, jobs, and revenues.[71][76]
A panel of experts who had previously participated in the IPCC reports have determined that it is not yet possible to determine a threshold for ocean acidity that should not be exceeded.[77]
Effects on calcification
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells and plates out of calcium carbonate (CaCO
3).[78] This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, such as coccoliths. After they are formed, such structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
Mechanism
Of the extra carbon dioxide added into the oceans, some remains as dissolved carbon dioxide, while the rest contributes towards making additional bicarbonate (and additional carbonic acid). This also increases the concentration of hydrogen ions, and the percentage increase in hydrogen is larger than the percentage increase in bicarbonate,[79] creating an imbalance in the reaction HCO3− ⇌ CO32− + H+. To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean's concentration of carbonate ions is reduced, creating an imbalance in the reaction Ca2+ + CO32− ⇌ CaCO3, and making the dissolution of formed CaCO
3 structures more likely.
The increase in concentrations of dissolved carbon dioxide and bicarbonate, and reduction in carbonate, are shown in a Bjerrum plot.
Saturation state
The saturation state (known as Ω) of seawater for a mineral is a measure of the thermodynamic potential for the mineral to form or to dissolve, and for calcium carbonate is described by the following equation:
Here Ω is the product of the concentrations (or activities) of the reacting ions that form the mineral (Ca2+
and CO2−
3), divided by the product of the concentrations of those ions when the mineral is at equilibrium (K
sp), that is, when the mineral is neither forming nor dissolving.[80] In seawater, a natural horizontal boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon.[78] Above this saturation horizon, Ω has a value greater than 1, and CaCO
3 does not readily dissolve. Most calcifying organisms live in such waters.[78] Below this depth, Ω has a value less than 1, and CaCO
3 will dissolve. However, if its production rate is high enough to offset dissolution, CaCO
3 can still occur where Ω is less than 1. The carbonate compensation depth occurs at the depth in the ocean where production is exceeded by dissolution.[81]
The decrease in the concentration of CO32− decreases Ω, and hence makes CaCO
3 dissolution more likely.
Calcium carbonate occurs in two common polymorphs (crystalline forms): aragonite and calcite. Aragonite is much more soluble than calcite, so the aragonite saturation horizon is always nearer to the surface than the calcite saturation horizon.[78] This also means that those organisms that produce aragonite may be more vulnerable to changes in ocean acidity than those that produce calcite.[35] Increasing CO
2 levels and the resulting lower pH of seawater decreases the saturation state of CaCO
3 and raises the saturation horizons of both forms closer to the surface.[82] This decrease in saturation state is believed to be one of the main factors leading to decreased calcification in marine organisms, as the inorganic precipitation of CaCO
3 is directly proportional to its saturation state.[83]
Impacts
Increasing acidity has possibly harmful consequences, such as depressing metabolic rates in jumbo squid,[84] depressing the immune responses of blue mussels,[85] and coral bleaching.
The reports "Ocean Acidification Summary for Policymakers 2013" and the IPCC approved "Special Report on the Ocean and Cryosphere in a Changing Climate" from 2019 describe research findings and possible impacts.[86][87]
Coral bleaching
The phenomenon of coral bleaching or coral whitening and the degeneration of coralline reef ecosystems is one consequence of increasing ocean acidity. The tropical and sub-tropical environments, including areas such as the Caribbean and surrounding regions, tropical Asia (e.g. Indonesia, Philippines, Thailand, Maldives) and the tropical Pacific (e.g. Australian Barrier Reef, Pacific Islands, Papua New Guinea) are mostly affected by coral bleaching, as these are the regions of the world that contain the largest and most extensive coral reef systems.[88]
Impacts on oceanic calcifying organisms

Increasing ocean acidification makes it more difficult for shell-accreting organisms to access carbonate ions, essential for the production of their hard exoskeletal shell.[29] Oceanic calcifying organism span the food chain from autotrophs to heterotrophs and include organisms such as coccolithophores, corals, foraminifera, echinoderms, crustaceans and molluscs.[71][89] As described above, under normal conditions, calcite and aragonite are stable in surface waters since the carbonate ion is at supersaturating concentrations. However, as ocean pH falls, the concentration of carbonate ions also decreases, and when carbonate becomes undersaturated, structures made of calcium carbonate are vulnerable to dissolution. Therefore, even if there is no change in the rate of calcification, the rate of dissolution of calcareous material increases.[90]

Corals,[91][92][93][94] coccolithophore algae,[95][96][97][98] coralline algae,[99] foraminifera,[100] shellfish[101] and pteropods[35][102] experience reduced calcification or enhanced dissolution when exposed to elevated CO
2.
The Royal Society published a comprehensive overview of ocean acidification, and its potential consequences, in June 2005.[78] However, some studies have found different responses to ocean acidification, with coccolithophore calcification and photosynthesis both increasing under elevated atmospheric pCO2,[103][104][105] an equal decline in primary production and calcification in response to elevated CO2[106] or the direction of the response varying between species.[107] A study in 2008 examining a sediment core from the North Atlantic found that while the species composition of coccolithophorids has remained unchanged for the industrial period 1780 to 2004, the calcification of coccoliths has increased by up to 40% during the same time.[105] A 2010 study from Stony Brook University suggested that while some areas are overharvested and other fishing grounds are being restored, because of ocean acidification it may be impossible to bring back many previous shellfish populations.[108] While the full ecological consequences of these changes in calcification are still uncertain, it appears likely that many calcifying species will be adversely affected.
When exposed in experiments to pH reduced by 0.2 to 0.4, larvae of a temperate brittlestar, a relative of the common sea star, fewer than 0.1 percent survived more than eight days.[57] There is also a suggestion that a decline in the coccolithophores may have secondary effects on climate, contributing to global warming by decreasing the Earth's albedo via their effects on oceanic cloud cover.[109] All marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes.[71]
The fluid in the internal compartments (the coelenteron) where corals grow their exoskeleton is also extremely important for calcification growth. When the saturation rate of aragonite in the external seawater is at ambient levels, the corals will grow their aragonite crystals rapidly in their internal compartments, hence their exoskeleton grows rapidly. If the level of aragonite in the external seawater is lower than the ambient level, the corals have to work harder to maintain the right balance in the internal compartment. When that happens, the process of growing the crystals slows down, and this slows down the rate of how much their exoskeleton is growing. Depending on how much aragonite is in the surrounding water, the corals may even stop growing because the levels of aragonite are too low to pump into the internal compartment. They could even dissolve faster than they can make the crystals to their skeleton, depending on the aragonite levels in the surrounding water.[110] Under the current progression of carbon emissions, around 70% of North Atlantic cold-water corals will be living in corrosive waters by 2050–60.[111]
A study conducted by the Woods Hole Oceanographic Institution in January 2018 showed that the skeletal growth of corals under acidified conditions is primarily affected by a reduced capacity to build dense exoskeletons, rather than affecting the linear extension of the exoskeleton. Using Global Climate Models, they show that the density of some species of corals could be reduced by over 20% by the end of this century.[112]
An in situ experiment on a 400 m2 patch of the Great Barrier Reef to decrease seawater CO2 level (raise pH) to close to the preindustrial value showed a 7% increase in net calcification.[113] A similar experiment to raise in situ seawater CO2 level (lower pH) to a level expected soon after the middle of this century found that net calcification decreased 34%.[114]
Ocean acidification may force some organisms to reallocate resources away from productive endpoints such as growth in order to maintain calcification.[115] For example, the oyster Magallana gigas is recognized to experience metabolic changes alongside altered calcification rates due to energetic tradeoffs resulting from pH imbalances.[116]
In some places carbon dioxide bubbles out from the sea floor, locally changing the pH and other aspects of the chemistry of the seawater. Studies of these carbon dioxide seeps have documented a variety of responses by different organisms.[2] Coral reef communities located near carbon dioxide seeps are of particular interest because of the sensitivity of some corals species to acidification. In Papua New Guinea, declining pH caused by carbon dioxide seeps is associated with declines in coral species diversity.[117] However, in Palau carbon dioxide seeps are not associated with reduced species diversity of corals, although bioerosion of coral skeletons is much higher at low pH sites.
Ocean acidification may affect the ocean's biologically driven sequestration of carbon from the atmosphere to the ocean interior and seafloor sediment, weakening the so-called biological pump.[118] Seawater acidification could also see Antarctic phytoplanktons smaller and less effective at storing carbon.[119] Such changes are being increasingly studied and synthesized through the use of physiological frameworks, including the Adverse Outcome Pathway (AOP) framework.[116]
Other biological impacts
Aside from the slowing and/or reversal of calcification, organisms may suffer other adverse effects, either indirectly through negative impacts on food resources,[78] or directly as reproductive or physiological effects. For example, the elevated oceanic levels of CO2 may produce CO
2-induced acidification of body fluids, known as hypercapnia. Also, increasing ocean acidity is believed to have a range of direct consequences. For example, increasing acidity has been observed to: reduce metabolic rates in jumbo squid;[84] depress the immune responses of blue mussels.[85] This is possibly because ocean acidification may alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise.[120] This impacts all animals that use sound for echolocation or communication.[121] Atlantic longfin squid eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH. The lower PH was simulated with 20–30 times the normal amount of CO2.[122] However, as with calcification, as yet there is not a full understanding of these processes in marine organisms or ecosystems.[123]
Another possible effect would be an increase in red tide events, which could contribute to the accumulation of toxins (domoic acid, brevetoxin, saxitoxin) in small organisms such as anchovies and shellfish, in turn increasing occurrences of amnesic shellfish poisoning, neurotoxic shellfish poisoning and paralytic shellfish poisoning.[124]
Although red tide is harmful, other beneficial photosynthetic organisms may benefit from increased levels of carbon dioxide. Most importantly, seagrasses will benefit.[125] An experiment done in 2018 concluded that as seagrasses increased their photosynthetic activity, calcifying algae's calcification rates rose. This could be a potential mitigation technique in the face of increasing acidity.[125]
Ocean acidification may benefit some species, for example increasing the growth rate of the sea star Pisaster ochraceus.[126]
Fish larvae
Ocean acidification can also have affects on marine fish larvae. It internally affects their olfactory systems, which is a crucial part of their development, especially in the beginning stage of their life. Orange clownfish larvae mostly live on oceanic reefs that are surrounded by vegetative islands.[127] With the use of their sense of smell, larvae are known to be able to detect the differences between reefs surrounded by vegetative islands and reefs not surrounded by vegetative islands.[127] Clownfish larvae need to be able to distinguish between these two destinations to have the ability to locate an area that is satisfactory for their growth. Another use for marine fish olfactory systems is to help in determining the difference between their parents and other adult fish in order to avoid inbreeding.
At James Cook University's experimental aquarium facility, clownfish were sustained in non-manipulated seawater that obtained a pH of 8.15 ± 0.07 which is similar to our current ocean's pH. To test for effects of different pH levels, seawater was manipulated to three different pH levels, including the non-manipulated pH. The two opposing pH levels correspond with climate change models that predict future atmospheric CO2 levels.[127] In the year 2100 the model predicts that we could potentially acquire CO2 levels at 1,000 ppm, which correlates with the pH of 7.8 ± 0.05. Results of this experiment show that when larvae is exposed to a pH of 7.8 ± 0.05 their reaction to environmental cues differs drastically to larvae's reaction to cues in a non-manipulated pH. At the pH of 7.6 ± 0.05 larvae had no reaction to any type of cue. These results display the negative outcomes that could possibly be the future for marine fish larvae. However, recent studies published in 2021 and 2022 suggest that the effects of ocean acidification on the behavior of fish larvae may not be universal or as severe as previously thought.[128][129]
Fish behavior
Multiple studies published in 2009 and 2010 reported drastic effects of ocean acidification on the behavior of coral fish.[130][131][132] This led to more than a decade of research regarding the effects of ocean acidification on animal behavior, including fish and invertebrates.[133] However, a study in 2020 challenged the potential negative impact of end-of-century ocean acidification level on the coral fish behavior, reporting that the results of the aforementioned studies from 2009 to 2010 were not replicable and suggesting that the effect of acidification on fish behavior could be negligible.[134] Furthermore, a meta-analysis published in 2022 found that the effect sizes of published studies testing for ocean acidification effects on fish behavior have declined by an order of magnitude over the past decade and have been negligible for the past five years, constituting a textbook example of the decline effect in science.[129]
Ecosystem impacts amplified by ocean warming and deoxygenation

While the full implications of elevated CO2 on marine ecosystems are still being documented, there is a substantial body of research showing that a combination of ocean acidification and elevated ocean temperature, driven mainly by CO2 and other greenhouse gas emissions, have a compounded effect on marine life and the ocean environment. This effect far exceeds the individual harmful impact of either.[137][138][139] In addition, ocean warming exacerbates ocean deoxygenation, which is an additional stressor on marine organisms, by increasing ocean stratification, through density and solubility effects, thus limiting nutrients,[140][141] while at the same time increasing metabolic demand.
Meta analyses have quantified the direction and magnitude of the harmful effects of ocean acidification, warming and deoxygenation on the ocean.[142][143][144] These meta-analyses have been further tested by mesocosm studies[145][146] that simulated the interaction of these stressors and found a catastrophic effect on the marine food web, i.e. that the increases in consumption from thermal stress more than negates any primary producer to herbivore increase from elevated CO2.
Nonbiological impacts
Leaving aside direct biological effects, it is expected that ocean acidification in the future will lead to a significant decrease in the burial of carbonate sediments for several centuries, and even the dissolution of existing carbonate sediments.[147] This will cause an elevation of ocean alkalinity, leading to the enhancement of the ocean as a reservoir for CO2 with implications for climate change as more CO2 leaves the atmosphere for the ocean.[148]
Impacts on human industry
The threat of acidification includes a decline in commercial fisheries and in the Arctic tourism industry and economy. Commercial fisheries are threatened because acidification harms calcifying organisms which form the base of the Arctic food webs.
Pteropods and brittle stars both form the base of the Arctic food webs and are both seriously damaged from acidification. Pteropods shells dissolve with increasing acidification and the brittle stars lose muscle mass when re-growing appendages.[149] For pteropods to create shells they require aragonite which is produced through carbonate ions and dissolved calcium. Pteropods are severely affected because increasing acidification levels have steadily decreased the amount of water supersaturated with carbonate which is needed for aragonite creation.[150] Arctic waters are changing so rapidly that they will become undersaturated with aragonite as early as 2016.[150] Additionally the brittle star's eggs die within a few days when exposed to expected conditions resulting from Arctic acidification.[151] Acidification threatens to destroy Arctic food webs from the base up. Arctic food webs are considered simple, meaning there are few steps in the food chain from small organisms to larger predators. For example, pteropods are "a key prey item of a number of higher predators – larger plankton, fish, seabirds, whales".[152] Both pteropods and sea stars serve as a substantial food source and their removal from the simple food web would pose a serious threat to the whole ecosystem. The effects on the calcifying organisms at the base of the food webs could potentially destroy fisheries. The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators.[153] Other organisms are directly harmed as a result of acidification. For example, decrease in the growth of marine calcifiers such as the American lobster, ocean quahog, and scallops means there is less shellfish meat available for sale and consumption.[154] Red king crab fisheries are also at a serious threat because crabs are calcifiers and rely on carbonate ions for shell development. Baby red king crab when exposed to increased acidification levels experienced 100% mortality after 95 days.[155] In 2006, red king crab accounted for 23% of the total guideline harvest levels and a serious decline in red crab population would threaten the crab harvesting industry.[156] Several ocean goods and services are likely to be undermined by future ocean acidification potentially affecting the livelihoods of some 400 to 800 million people depending upon the emission scenario.[71]
Impacts on indigenous peoples
Acidification could damage the Arctic tourism economy and affect the way of life of indigenous peoples. A major pillar of Arctic tourism is the sport fishing and hunting industry. The sport fishing industry is threatened by collapsing food webs which provide food for the prized fish. A decline in tourism lowers revenue input in the area, and threatens the economies that are increasingly dependent on tourism.[157] The rapid decrease or disappearance of marine life could also affect the diet of Indigenous peoples.
Possible responses
.jpg.webp)
Reducing greenhouse gas emissions
Members of the InterAcademy Panel recommended that by 2050, global anthropogenic CO2 emissions be reduced less than 50% of the 1990 level.[14] The 2009[14] statement also called on world leaders to:
- Acknowledge that ocean acidification is a direct and real consequence of increasing atmospheric CO2 concentrations, is already having an effect at current concentrations, and is likely to cause grave harm to important marine ecosystems as CO2 concentrations reach 450 [parts-per-million (ppm)] and above;
- ... Recognize that reducing the build up of CO2 in the atmosphere is the only practicable solution to mitigating ocean acidification;
- ... Reinvigorate action to reduce stressors, such as overfishing and pollution, on marine ecosystems to increase resilience to ocean acidification.[158]
Stabilizing atmospheric CO2 concentrations at 450 ppm would require near-term emissions reductions, with steeper reductions over time.[159]
The German Advisory Council on Global Change[160] stated:
In order to prevent disruption of the calcification of marine organisms and the resultant risk of fundamentally altering marine food webs, the following guard rail should be obeyed: the pH of near surface waters should not drop more than 0.2 units below the pre-industrial average value in any larger ocean region (nor in the global mean).
One policy target related to ocean acidity is the magnitude of future global warming. Parties to the United Nations Framework Convention on Climate Change (UNFCCC) adopted a target of limiting warming to below 2 °C, relative to the pre-industrial level.[161] Meeting this target would require substantial reductions in anthropogenic CO2 emissions.[162]
Limiting global warming to below 2 °C would imply a reduction in surface ocean pH of 0.16 from pre-industrial levels. This would represent a substantial decline in surface ocean pH.[163]
On 25 September 2015, USEPA denied[164] a 30 June 2015, citizens petition[165] that asked EPA to regulate CO2 under TSCA in order to mitigate ocean acidification. In the denial, EPA said that risks from ocean acidification were being "more efficiently and effectively addressed" under domestic actions, e.g., under the Presidential Climate Action Plan,[166] and that multiple avenues are being pursued to work with and in other nations to reduce emissions and deforestation and promote clean energy and energy efficiency.
On 28 March 2017 the US by executive order rescinded the Climate Action Plan.[167] On 1 June 2017 it was announced the US would withdraw from the Paris accords,[168] and on 12 June 2017 that the US would abstain from the G7 Climate Change Pledge,[169] two major international efforts to reduce CO2 emissions.
On 14 September 2022 USEPA denied [170]| a citizens petition [171] | on behalf of individual petitioners: Donn Viviani, James Hansen, John Birk, Richard Heede, and Lise Van Sustren, and the two non-profit organizations: the Climate Protection and Restoration Initiative and Climate Science, Awareness and Solutions, to regulate CO2 and other greenhouse gases and prevent the unreasonable effects on the environment from ocean acidification.
Other solutions such as increasing the land devoted to forests and encouraging the growth of CO2-breathing sea plants can mitigate ocean acidification.[172]
Geoengineering
Geoengineering has been proposed as a possible response to ocean acidification. The IAP (2009)[14] statement said more research is needed to prove that this would be safe, affordable and worthwhile:
Mitigation approaches such as adding chemicals to counter the effects of acidification are likely to be expensive, only partly effective and only at a very local scale, and may pose additional unanticipated risks to the marine environment. There has been very little research on the feasibility and impacts of these approaches. Substantial research is needed before these techniques could be applied.
Reports by the WGBU (2006),[160] the UK's Royal Society (2009),[173] and the US National Research Council (2011)[174] warned of the potential risks and difficulties associated with climate engineering.
Iron fertilization
Iron fertilization of the ocean could stimulate photosynthesis in phytoplankton (see Iron hypothesis). The phytoplankton would convert the ocean's dissolved carbon dioxide into carbohydrate and oxygen gas, some of which would sink into the deeper ocean before oxidizing. More than a dozen open-sea experiments confirmed that adding iron to the ocean increases photosynthesis in phytoplankton by up to 30 times.[175] While this approach has been proposed as a potential solution to the ocean acidification problem, mitigation of surface ocean acidification might increase acidification in the less-inhabited deep ocean.[176]
A report by the UK's Royal Society (2009)[177] reviewed the approach for effectiveness, affordability, timeliness and safety. The rating for affordability was "medium", or "not expected to be very cost-effective". For the other three criteria, the ratings ranged from "low" to "very low" (i.e., not good). For example, in regards to safety, the report found a "[high] potential for undesirable ecological side effects", and that ocean fertilization "may increase anoxic regions of ocean ('dead zones')".[178]
Global goals

The problem of ocean acidification is included in one of the targets of the United Nations' Sustainable Development Goal 14:[15] Ocean acidification is directly addressed by the target SDG 14.3. The full title of Target 14.3 is: "Minimize and address the impacts of ocean acidification, including through enhanced scientific cooperation at all levels".[180] This target has one indicator: Indicator 14.3.1 is the "Average marine acidity (pH) measured at agreed suite of representative sampling stations".[181]
Ocean acidification in the geologic past
Ocean acidification has occurred previously in Earth's history,[182] and the resulting ecological collapse in the oceans had long-lasting effects on global carbon cycling and climate.[183][184] The most notable example is the Paleocene–Eocene Thermal Maximum (PETM),[185] which occurred approximately 56 million years ago when massive amounts of carbon entered the ocean and atmosphere, and led to the dissolution of carbonate sediments in all ocean basins.
Three of the big five mass extinction events in the geologic past were associated with a rapid increase in atmospheric carbon dioxide, probably due to volcanism and/or thermal dissociation of marine gas hydrates.[186][187] Early research focused on the climatic effects of the elevated CO2 levels on biodiversity,[188] but in 2004, decreased CaCO3 saturation due to seawater uptake of volcanogenic CO2 was suggested as a possible kill mechanism during the marine mass extinction at the end of the Triassic.[189] The end-Triassic biotic crisis is still the most well-established example of a marine mass extinction due to ocean acidification, because (a) volcanic activity, changes in carbon isotopes, decrease of carbonate sedimentation, and marine extinction coincided precisely in the stratigraphic record,[190][191][192][193] and (b) there was pronounced selectivity of the extinction against organisms with thick aragonitic skeletons,[190][194][195] which is predicted from experimental studies.[91][92][196][197] Ocean acidification has also been suggested as a cause of the end-Permian mass extinction[198][199] and the end-Cretaceous crisis.[200]
Gallery
 "Present day" (1990s) sea surface pH
"Present day" (1990s) sea surface pH Present day alkalinity
Present day alkalinity "Present day" (1990s) sea surface anthropogenic CO
"Present day" (1990s) sea surface anthropogenic CO
2 Vertical inventory of "present day" (1990s) anthropogenic CO
Vertical inventory of "present day" (1990s) anthropogenic CO
2 Change in surface CO2−
Change in surface CO2−
3 ion from the 1700s to the 1990s Present day DIC
Present day DIC Pre-Industrial DIC
Pre-Industrial DIC A NOAA (AOML) in situ CO
A NOAA (AOML) in situ CO
2 concentration sensor (SAMI-CO2), attached to a Coral Reef Early Warning System station, utilized in conducting ocean acidification studies near coral reef areas A NOAA (PMEL) moored autonomous CO
A NOAA (PMEL) moored autonomous CO
2 buoy used for measuring CO
2 concentration and ocean acidification studies
See also
- Biological pump – Carbon capture process in oceans
- Free Ocean CO2 Enrichment - technology for studying ocean acidification
- Carbon sink – Reservoir absorbing more carbon from than emitting to the air, storing carbon over the long term
- Estuarine acidification – Compex Process of pH change
- Holocene extinction – Ongoing extinction event caused by human activity
- Ocean acidification in the Arctic Ocean
- Ocean acidification in the Great Barrier Reef – Threat to the reef which reduces the viability and strength of reef-building corals
- Ocean deoxygenation – Reduction of the oxygen content of the oceans
- Ocean storage of carbon dioxide – Possible method of carbon sequestration
- Water pollution
References
- Jacobson, M. Z. (2005). "Studying ocean acidification with conservative, stable numerical schemes for nonequilibrium air-ocean exchange and ocean equilibrium chemistry". Journal of Geophysical Research: Atmospheres. 110: D07302. Bibcode:2005JGRD..11007302J. doi:10.1029/2004JD005220.
- Hall-Spencer, J. M.; Rodolfo-Metalpa, R.; Martin, S.; et al. (July 2008). "Volcanic carbon dioxide vents show ecosystem effects of ocean acidification". Nature. 454 (7200): 96–9. Bibcode:2008Natur.454...96H. doi:10.1038/nature07051. hdl:10026.1/1345. PMID 18536730. S2CID 9375062.
- Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Hauck, Judith; Olsen, Are; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Alin, Simone (11 December 2020). "Global Carbon Budget 2020". Earth System Science Data. 12 (4): 3269–3340. Bibcode:2020ESSD...12.3269F. doi:10.5194/essd-12-3269-2020. ISSN 1866-3508.
- Field, C.B.; Barros, V; Stocker, T.F.; Dahe, Q; et al. "IPCC Workshop on Ocean Acidification on Marine Biology and Ecosystems — IPCC". IPCC Workshop on Ocean Acidification on Marine Biology and Ecosystems. Retrieved 28 September 2022.
{{cite web}}: CS1 maint: url-status (link) - Robert E. Service (13 July 2012). "Rising Acidity Brings and Ocean Of Trouble". Science. 337 (6091): 146–148. Bibcode:2012Sci...337..146S. doi:10.1126/science.337.6091.146. PMID 22798578.
- Cornelia Dean (30 January 2009). "Rising Acidity Is Threatening Food Web of Oceans, Science Panel Says". New York Times.
- Boudreau, Bernard P.; Middelburg, Jack J.; Luo, Yiming (30 November 2018). "The role of calcification in carbonate compensation". Nature Geoscience. 11 (12): 894–900. Bibcode:2018NatGe..11..894B. doi:10.1038/s41561-018-0259-5. ISSN 1752-0908. S2CID 135284130.
- "Report of the Ocean Acidification and Oxygen Working Group, International Council for Science's Scientific Committee on Ocean Research (SCOR) Biological Observatories Workshop" (PDF).
- Osborne, Emily B.; Thunell, Robert C.; Gruber, Nicolas; Feely, Richard A.; Benitez-Nelson, Claudia R. (16 December 2019). "Decadal variability in twentieth-century ocean acidification in the California Current Ecosystem". Nature Geoscience. 13 (1): 43–49. doi:10.1038/s41561-019-0499-z. ISSN 1752-0908. S2CID 209381004.
- Wallace, Ryan B.; Baumann, Hannes; Grear, Jason S.; Aller, Robert C.; Gobler, Christopher J. (5 July 2014). "Coastal ocean acidification: The other eutrophication problem". Estuarine, Coastal and Shelf Science. 148: 1–13. Bibcode:2014ECSS..148....1W. doi:10.1016/j.ecss.2014.05.027. ISSN 0272-7714.
- Doney, Scott C.; Mahowald, Natalie; Lima, Ivan; Feely, Richard A.; Mackenzie, Fred T.; Lamarque, Jean-Francois; Rasch, Phil J. (11 September 2007). "Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system". Proceedings of the National Academy of Sciences. 104 (37): 14580–14585. Bibcode:2007PNAS..10414580D. doi:10.1073/pnas.0702218104. ISSN 0027-8424. PMC 1965482. PMID 17804807.
- Anthony, KRN; et al. (2008). "Ocean acidification causes bleaching and productivity loss in coral reef builders". Proceedings of the National Academy of Sciences. 105 (45): 17442–17446. Bibcode:2008PNAS..10517442A. doi:10.1073/pnas.0804478105. PMC 2580748. PMID 18988740.
- Robert E. Service (13 July 2012). "Rising Acidity Brings and Ocean Of Trouble". Science. 337 (6091): 146–148. Bibcode:2012Sci...337..146S. doi:10.1126/science.337.6091.146. PMID 22798578.
- IAP (June 2009). "Interacademy Panel (IAP) Member Academies Statement on Ocean Acidification"., Secretariat: TWAS (the Academy of Sciences for the Developing World), Trieste, Italy.
- "Goal 14 targets". UNDP. Retrieved 24 September 2020.
- Feely, R. A.; Sabine, C. L.; Hernandez-Ayon, J. M.; Ianson, D.; Hales, B. (June 2008). "Evidence for upwelling of corrosive "acidified" water onto the continental shelf". Science. 320 (5882): 1490–2. Bibcode:2008Sci...320.1490F. CiteSeerX 10.1.1.328.3181. doi:10.1126/science.1155676. PMID 18497259. S2CID 35487689. Retrieved 25 January 2014 – via Pacific Marine Environmental Laboratory (PMEL).
- Raven, J. A.; Falkowski, P. G. (1999). "Oceanic sinks for atmospheric CO2". Plant, Cell & Environment. 22 (6): 741–755. doi:10.1046/j.1365-3040.1999.00419.x.
- Friedlingstein, Pierre; Jones, Matthew W.; O'Sullivan, Michael; Andrew, Robbie M.; et al. (26 April 2022). "Global Carbon Budget 2021". Earth System Science Data. 14 (4): 1917–2005. Bibcode:2022ESSD...14.1917F. doi:10.5194/essd-14-1917-2022. ISSN 1866-3508.
- "carbon cycle". Encyclopædia Britannica Online. Retrieved 11 February 2010.
- Kump, Lee R.; Kasting, James F.; Crane, Robert G. (2003). The Earth System (2nd ed.). Upper Saddle River: Prentice Hall. pp. 162–164. ISBN 978-0-613-91814-5.
- Nina Notman (29 July 2014). "The other carbon dioxide problem". Chemistry World.
- Alex Rogers (9 October 2013). "Global warming's evil twin: ocean acidification". The Conversation.
- "Ocean acidification (Issues Brief)" (PDF). IUCN (International Union for Conservation of Nature). November 2017. Retrieved 3 November 2020.
- Gies, E. (11 January 2018). "Like Oceans, Freshwater Is Also Acidifying". Scientific American. Retrieved 13 January 2018.
- Weiss, L. C.; Pötter, L.; Steiger, A.; Kruppert, S.; Frost, U.; Tollrian, R. (2018). "Rising pCO2 in Freshwater Ecosystems Has the Potential to Negatively Affect Predator-Induced Defenses in Daphnia". Current Biology. 28 (2): 327–332.e3. doi:10.1016/j.cub.2017.12.022. PMID 29337079.
- Millero, Frank J. (1995). "Thermodynamics of the carbon dioxide system in the oceans". Geochimica et Cosmochimica Acta. 59 (4): 661–677. Bibcode:1995GeCoA..59..661M. doi:10.1016/0016-7037(94)00354-O.
- Feely, R. A.; Sabine, C. L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V. J.; Millero, F. J. (July 2004). "Impact of Anthropogenic CO2on the CaCO3 System in the Oceans". Science. 305 (5682): 362–366. Bibcode:2004Sci...305..362F. doi:10.1126/science.1097329. PMID 15256664. S2CID 31054160. Retrieved 25 January 2014 – via Pacific Marine Environmental Laboratory (PMEL).
- Paul Freund; Stefan Bachu; Dale Simbeck; Kelly (Kailai) Thambimuthu; Murlidhar Gupta (2005). "Annex I: Properties of CO2 and carbon-based fuels". In Bert Metz; Ogunlade Davidson; Heleen de Coninck; Manuela Loos; Leo Meyer (eds.). IPCC Special Report on Carbon Dioxide Capture and Storage (PDF). IPCC. p. 390. Archived from the original (PDF) on 10 February 2010. Retrieved 1 November 2014.
- "PMEL CO2 - Carbon Dioxide Program". www.pmel.noaa.gov. Retrieved 6 September 2021.
- Lee, J.Y.; Marotzke, C.; Bala, L.; Cao, S.; et al. (2021). "Future Global Climate: Scenario-Based Projections and Near- Term Information" (PDF). In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (PDF). Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press. p. 608. doi:10.1017/9781009157896.006 (inactive 24 October 2022).
{{cite book}}: CS1 maint: DOI inactive as of October 2022 (link) - Brander, Luke M.; Rehdanz, Katrin; Tol, Richard S. J.; Van Beukering, Pieter J. H. (1 February 2012). "The economic impact of ocean acidification on coral reefs". Climate Change Economics. 03 (1): 1250002. doi:10.1142/S2010007812500029. hdl:2262/27779. ISSN 2010-0078.
- Cooley, S. R., D. J. P. Moore, S. R. Alin, D. Butman, D. W. Clow, N. H. F. French, R. A. Feely, Z. I. Johnson, G. Keppel-Aleks, S. E. Lohrenz, I. B. Ocko, E. H. Shadwick, A. J. Sutton, C. S. Potter, Y. Takatsuka, A. P. Walker, and R. M. S. Yu, 2018: Chapter 17: Biogeochemical effects of rising atmospheric carbon dioxide. In Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report [Cavallaro, N., G. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, pp. 690-727, https://doi.org/10.7930/SOCCR2.2018.Ch17.
- Orr, James C.; Fabry, Victoria J.; Aumont, Olivier; Bopp, Laurent; Doney, Scott C.; Feely, Richard A.; Gnanadesikan, Anand; Gruber, Nicolas; Ishida, Akio; Joos, Fortunat; Key, Robert M.; Lindsay, Keith; Maier-Reimer, Ernst; Matear, Richard; Monfray, Patrick (September 2005). "Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms". Nature. 437 (7059): 681–686. Bibcode:2005Natur.437..681O. doi:10.1038/nature04095. ISSN 0028-0836. PMID 16193043. S2CID 4306199.
- Feely, Richard A.; Sabine, Christopher L.; Hernandez-Ayon, J. Martin; Ianson, Debby; Hales, Burke (13 June 2008). "Evidence for Upwelling of Corrosive "Acidified" Water onto the Continental Shelf". Science. 320 (5882): 1490–1492. Bibcode:2008Sci...320.1490F. doi:10.1126/science.1155676. ISSN 0036-8075. PMID 18497259. S2CID 35487689.
- Orr, James C.; et al. (2005). "Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms" (PDF). Nature. 437 (7059): 681–686. Bibcode:2005Natur.437..681O. doi:10.1038/nature04095. PMID 16193043. S2CID 4306199. Archived from the original (PDF) on 25 June 2008.
- Key, R. M.; Kozyr, A.; Sabine, C. L.; Lee, K.; Wanninkhof, R.; Bullister, J.; Feely, R. A.; Millero, F.; Mordy, C.; Peng, T.-H. (2004). "A global ocean carbon climatology: Results from GLODAP". Global Biogeochemical Cycles. 18 (4): GB4031. Bibcode:2004GBioC..18.4031K. doi:10.1029/2004GB002247. S2CID 16428889.

- "Ocean acidification and the Southern Ocean". Australian Antarctic Division — Australia in Antarctica.
- "EPA weighs action on ocean acidification". 4 February 2009.
- Review of Past IPCC Emissions Scenarios, IPCC Special Report on Emissions Scenarios (ISBN 0521804930).
- Carstensen, Jacob; Duarte, Carlos M. (16 April 2019). "Drivers of pH Variability in Coastal Ecosystems". Environmental Science & Technology. 53 (8): 4020–4029. Bibcode:2019EnST...53.4020C. doi:10.1021/acs.est.8b03655. ISSN 0013-936X. PMID 30892892. S2CID 84841808.
- Duarte, Carlos M.; Hendriks, Iris E.; Moore, Tommy S.; Olsen, Ylva S.; Steckbauer, Alexandra; Ramajo, Laura; Carstensen, Jacob; Trotter, Julie A.; McCulloch, Malcolm (1 March 2013). "Is Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater pH". Estuaries and Coasts. 36 (2): 221–236. doi:10.1007/s12237-013-9594-3. ISSN 1559-2731.
- Lowe, Alexander T.; Bos, Julia; Ruesink, Jennifer (30 January 2019). "Ecosystem metabolism drives pH variability and modulates long-term ocean acidification in the Northeast Pacific coastal ocean". Scientific Reports. 9 (1): 963. Bibcode:2019NatSR...9..963L. doi:10.1038/s41598-018-37764-4. ISSN 2045-2322. PMC 6353961. PMID 30700764.
- Fairchild, William; Hales, Burke (2021). "High-Resolution Carbonate System Dynamics of Netarts Bay, OR From 2014 to 2019". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.590236. ISSN 2296-7745.
- Cited in Tim Flannery, Atmosphere of Hope. Solutions to the Climate Crisis, Penguin Books, 2015, page 47 (ISBN 9780141981048).
- Wootton, J. T.; Pfister, C. A.; Forester, J. D. (2008). "Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset". Proceedings of the National Academy of Sciences. 105 (48): 18848–18853. Bibcode:2008PNAS..10518848W. doi:10.1073/pnas.0810079105. PMC 2596240. PMID 19033205.
- "Ocean Growing More Acidic Faster Than Once Thought; Increasing Acidity Threatens Sea Life". Science Daily. 26 November 2008. Retrieved 26 November 2008.
- McInerney, Francesca A.; Wing, Scott L. (30 May 2011). "The Paleocene-Eocene Thermal Maximum: A Perturbation of Carbon Cycle, Climate, and Biosphere with Implications for the Future". Annual Review of Earth and Planetary Sciences. 39 (1): 489–516. Bibcode:2011AREPS..39..489M. doi:10.1146/annurev-earth-040610-133431. ISSN 0084-6597.
- Zeebe, Richard E. (30 May 2012). "History of Seawater Carbonate Chemistry, Atmospheric CO 2 , and Ocean Acidification". Annual Review of Earth and Planetary Sciences. 40 (1): 141–165. Bibcode:2012AREPS..40..141Z. doi:10.1146/annurev-earth-042711-105521. ISSN 0084-6597.
- Joel, Lucas (21 October 2019). "The Dinosaur-Killing Asteroid Acidified the Ocean in a Flash - The Chicxulub event was as damaging to life in the oceans as it was to creatures on land, a study shows". The New York Times. Retrieved 22 October 2019.
- Henehan, Michael J.; et al. (21 October 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences of the United States of America. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. PMC 6842625. PMID 31636204.
- "An Ominous Warning on the Effects of Ocean Acidification by Carl Zimmer: Yale Environment 360". e360.yale.edu. Archived from the original on 16 February 2014. Retrieved 25 January 2014.
- Newspapers, Les Blumenthal-McClatchy (22 April 2010). "Report: Ocean acidification rising at unprecedented rate". mcclatchydc.
- United States National Research Council, 2010. Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean
- "The Geological Record of Ocean Acidification". JournalistsResource.org, retrieved 14 March 2012
- Hönisch, Bärbel; Ridgwell, Andy; Schmidt, Daniela N.; Thomas, E.; Gibbs, S. J.; Sluijs, A.; Zeebe, R.; Kump, L.; Martindale, R. C.; Greene, S. E.; Kiessling, W.; Ries, J.; Zachos, J. C.; Royer, D. L.; Barker, S.; Marchitto, T. M.; Moyer, R.; Pelejero, C.; Ziveri, P.; Foster, G. L.; Williams, B. (2012). "The Geological Record of Ocean Acidification". Science. 335 (6072): 1058–1063. Bibcode:2012Sci...335.1058H. doi:10.1126/science.1208277. hdl:1983/24fe327a-c509-4b6a-aa9a-a22616c42d49. PMID 22383840. S2CID 6361097.
- David (2 July 2005). "The Acid Ocean – the Other Problem with CO2 Emission". Real Climate.
- Marah J. Hardt; Carl Safina (9 August 2010). "How Acidification Threatens Oceans from the Inside Out". Scientific American. Archived from the original on 26 December 2010.
- "Ocean Acidification Is Climate Change's 'Equally Evil Twin,' NOAA Chief Says". Huffington Post. 9 July 2012. Archived from the original on 12 July 2012. Retrieved 9 July 2012.
- Fiona Harvey (25 August 2013). "Rising levels of acids in seas may endanger marine life, says study". The Guardian. Retrieved 29 August 2013.
- Harrabin, Roger (3 July 2015). "CO2 emissions threaten ocean crisis". BBC News.
- Humphreys, M. P. (2016). "Climate sensitivity and the rate of ocean acidification: future impacts, and implications for experimental design". ICES Journal of Marine Science. 74 (4): 934–940. doi:10.1093/icesjms/fsw189.
- Gulev, S.K., P.W. Thorne, J. Ahn, F.J. Dentener, C.M. Domingues, S. Gerland, D. Gong, D.S. Kaufman, H.C. Nnamchi, J. Quaas, J.A. Rivera, S. Sathyendranath, S.L. Smith, B. Trewin, K. von Schuckmann, and R.S. Vose, 2021: Changing State of the Climate System. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 287–422, doi:10.1017/9781009157896.004.
- Olafsson, J.; Olafsdottir, S. R.; Benoit-Cattin, A.; Danielsen, M.; Arnarson, T. S.; Takahashi, T. (25 November 2009). "Rate of Iceland Sea acidification from time series measurements". Biogeosciences. 6 (11): 2661–2668. Bibcode:2009BGeo....6.2661O. doi:10.5194/bg-6-2661-2009.
- Midorikawa, Takashi; Inoue, Hisayuki Y.; Ishii, Masao; Sasano, Daisuke; Kosugi, Naohiro; Hashida, Gen; Nakaoka, Shin-ichiro; Suzuki, Toru (March 2012). "Decreasing pH trend estimated from 35-year time series of carbonate parameters in the Pacific sector of the Southern Ocean in summer". Deep Sea Research Part I: Oceanographic Research Papers. 61: 131–139. Bibcode:2012DSRI...61..131M. doi:10.1016/j.dsr.2011.12.003.
- González-Dávila, M.; Santana-Casiano, J. M.; Rueda, M. J.; Llinás, O. (11 October 2010). "The water column distribution of carbonate system variables at the ESTOC site from 1995 to 2004". Biogeosciences. 7 (10): 3067–3081. Bibcode:2010BGeo....7.3067G. doi:10.5194/bg-7-3067-2010.
- Dore, J. E.; Lukas, R.; Sadler, D. W.; Church, M. J.; Karl, D. M. (28 July 2009). "Physical and biogeochemical modulation of ocean acidification in the central North Pacific". Proceedings of the National Academy of Sciences. 106 (30): 12235–12240. doi:10.1073/pnas.0906044106. PMC 2716384. PMID 19666624.
- Bates, N. R.; Best, M. H. P.; Neely, K.; Garley, R.; Dickson, A. G.; Johnson, R. J. (11 July 2012). "Detecting anthropogenic carbon dioxide uptake and ocean acidification in the North Atlantic Ocean". Biogeosciences. 9 (7): 2509–2522. Bibcode:2012BGeo....9.2509B. doi:10.5194/bg-9-2509-2012.
- Pelejero, Carles; Calvo, Eva; McCulloch, Malcolm T.; Marshall, John F.; Gagan, Michael K.; Lough, Janice M.; Opdyke, Bradley N. (30 September 2005). "Preindustrial to Modern Interdecadal Variability in Coral Reef pH". Science. 309 (5744): 2204–2207. Bibcode:2005Sci...309.2204P. doi:10.1126/science.1113692. PMID 16195458. S2CID 129883047.
- Bialik, Or M.; Sisma-Ventura, Guy (December 2016). "Proxy-based reconstruction of surface water acidification and carbonate saturation of the Levant Sea during the Anthropocene". Anthropocene. 16: 42–53. doi:10.1016/j.ancene.2016.08.001.
- Mora, Camilo; Frazier, Abby G.; Longman, Ryan J.; Dacks, Rachel S.; Walton, Maya M.; Tong, Eric J.; Sanchez, Joseph J.; Kaiser, Lauren R.; Stender, Yuko O.; Anderson, James M.; Ambrosino, Christine M. (10 October 2013). "The projected timing of climate departure from recent variability". Nature. 502 (7470): 183–187. Bibcode:2013Natur.502..183M. doi:10.1038/nature12540. ISSN 0028-0836. PMID 24108050. S2CID 4471413.
- Mora, C.; et al. (2013). "Biotic and Human Vulnerability to Projected Changes in Ocean Biogeochemistry over the 21st Century". PLOS Biology. 11 (10): e1001682. doi:10.1371/journal.pbio.1001682. PMC 3797030. PMID 24143135.
- Lee, J.-Y., J. Marotzke, G. Bala, L. Cao, S. Corti, J.P. Dunne, F. Engelbrecht, E. Fischer, J.C. Fyfe, C. Jones, A. Maycock, J. Mutemi, O. Ndiaye, S. Panickal, and T. Zhou, 2021: Future Global Climate: Scenario-Based Projections and Near- Term Information. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 553–672, doi:10.1017/9781009157896.006.
- Sosdian, S. M.; Lear, C. H. (2020). "Initiation of the Western Pacific Warm Pool at the Middle Miocene Climate Transition?". Paleoceanography and Paleoclimatology. 35 (12): 3920. Bibcode:2020PaPa...35.3920S. doi:10.1029/2020PA003920. ISSN 2572-4517. S2CID 228828002.
- McNeil BI; Matear RJ (2 December 2008), "Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2", Proceedings of the National Academy of Sciences, 105 (48): 18860–4, Bibcode:2008PNAS..10518860M, doi:10.1073/pnas.0806318105, PMC 2596239, PMID 19022908
- "UN: Oceans are 30 percent more acidic than before fossil fuels". Archived from the original on 3 January 2011.
- "What is Ocean Acidification". NOAA. Retrieved 24 August 2013.
- Gattuso, Jean-Pierre; Mach, Katharine J.; Morgan, Granger (April 2013). "Ocean acidification and its impacts: an expert survey". Climatic Change. 117 (4): 725–738. Bibcode:2013ClCh..117..725G. doi:10.1007/s10584-012-0591-5. ISSN 0165-0009. S2CID 153892043.
- Raven, JA, et al. (2005) "Ocean acidification due to increasing atmospheric carbon dioxide". Royal Society, London, UK.
- Mitchell, M. J.; et al. (2010). "A model of carbon dioxide dissolution and mineral carbonation kinetics". Proceedings of the Royal Society A. 466 (2117): 1265–1290. Bibcode:2010RSPSA.466.1265M. doi:10.1098/rspa.2009.0349.
- Atkinson, M.J.; Cuet, P. (2008). "Possible effects of ocean acidification on coral reef biogeochemistry: topics for research". Marine Ecology Progress Series. 373: 249–256. Bibcode:2008MEPS..373..249A. doi:10.3354/meps07867.
- Thurman, H.V.; Trujillo, A.P. (2004). Introductory Oceanography. Prentice Hall. ISBN 978-0-13-143888-0.
- The Royal Society. Ocean Acidification Due To Increasing Atmospheric Carbon Dioxide, The Clyvedon Press Ltd. (2005): 11.
- Marubini, F.; Ferrier-Pagès, C.; Furla, P.; Allemand, D. (2008). "Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism". Coral Reefs. 27 (3): 491–499. Bibcode:2008CorRe..27..491M. doi:10.1007/s00338-008-0375-6.
- Rosa, R.; Seibel, B. (2008). "Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator". PNAS. 105 (52): 20776–20780. Bibcode:2008PNAS..10520776R. doi:10.1073/pnas.0806886105. PMC 2634909. PMID 19075232.
- Bibby, R.; et al. (2008). "Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis". Aquatic Biology. 2: 67–74. doi:10.3354/ab00037.
- "Ocean Acidification Summary for Policymakers". IGBP.
- "Special Report on the Ocean and Cryosphere in a Changing Climate — Special Report on the Ocean and Cryosphere in a Changing Climate". IPCC. 25 September 2019. Retrieved 12 November 2019.
- Day, Jon C.; Heron, Scott F. "'Severely threatened and deteriorating': global authority on nature lists the Great Barrier Reef as critical". The Conversation. Retrieved 3 September 2021.
- National Research Council. Overview of Climate Changes and Illustrative Impacts. Climate Stabilization Targets: Emissions, Concentrations, and Impacts over Decades to Millennia. Washington, DC: The National Academies Press, 2011. 1. Print.
- Nienhuis, S.; Palmer, A.; Harley, C. (2010). "Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail". Proceedings of the Royal Society B. 277 (1693): 2553–2558. doi:10.1098/rspb.2010.0206. PMC 2894921. PMID 20392726.
- Gattuso, J.-P.; Frankignoulle, M.; Bourge, I.; Romaine, S.; Buddemeier, R. W. (1998). "Effect of calcium carbonate saturation of seawater on coral calcification". Global and Planetary Change. 18 (1–2): 37–46. Bibcode:1998GPC....18...37G. doi:10.1016/S0921-8181(98)00035-6.
- Gattuso, J.-P.; Allemand, D.; Frankignoulle, M. (1999). "Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry". American Zoologist. 39: 160–183. doi:10.1093/icb/39.1.160.
- Langdon, C.; Atkinson, M. J. (2005). "Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment". Journal of Geophysical Research. 110 (C09S07): C09S07. Bibcode:2005JGRC..11009S07L. doi:10.1029/2004JC002576.
- D'Olivo, Juan P.; Ellwood, George; DeCarlo, Thomas M.; McCulloch, Malcolm T. (15 November 2019). "Deconvolving the long-term impacts of ocean acidification and warming on coral biomineralisation". Earth and Planetary Science Letters. 526: 115785. Bibcode:2019E&PSL.52615785D. doi:10.1016/j.epsl.2019.115785. ISSN 0012-821X.
- Riebesell, Ulf; Zondervan, Ingrid; Rost, Björn; Tortell, Philippe D.; Zeebe, Richard E.; Morel, François M. M. (2000). "Reduced calcification of marine plankton in response to increased atmospheric CO
2" (PDF). Nature. 407 (6802): 364–367. Bibcode:2000Natur.407..364R. doi:10.1038/35030078. PMID 11014189. S2CID 4426501. - Zondervan, I.; Zeebe, R. E.; Rost, B.; Rieblesell, U. (2001). "Decreasing marine biogenic calcification: a negative feedback on rising atmospheric CO2" (PDF). Global Biogeochemical Cycles. 15 (2): 507–516. Bibcode:2001GBioC..15..507Z. doi:10.1029/2000GB001321.
- Zondervan, I.; Rost, B.; Rieblesell, U. (2002). "Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light limiting conditions and different day lengths" (PDF). Journal of Experimental Marine Biology and Ecology. 272 (1): 55–70. doi:10.1016/S0022-0981(02)00037-0.
- Delille, B.; Harlay, J.; Zondervan, I.; Jacquet, S.; Chou, L.; Wollast, R.; Bellerby, R.G.J.; Frankignoulle, M.; Borges, A.V.; Riebesell, U.; Gattuso, J.-P. (2005). "Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi". Global Biogeochemical Cycles. 19 (2): GB2023. Bibcode:2005GBioC..19.2023D. doi:10.1029/2004GB002318.
- Kuffner, I. B.; Andersson, A. J.; Jokiel, P. L.; Rodgers, K. S.; Mackenzie, F. T. (2007). "Decreased abundance of crustose coralline algae due to ocean acidification". Nature Geoscience. 1 (2): 114–117. Bibcode:2008NatGe...1..114K. doi:10.1038/ngeo100. S2CID 3456369.
- Phillips, Graham; Chris Branagan (13 September 2007). "Ocean Acidification – The BIG global warming story". ABC TV Science: Catalyst. Australian Broadcasting Corporation. Retrieved 18 September 2007.
- Gazeau, F.; Quiblier, C.; Jansen, J. M.; Gattuso, J.-P.; Middelburg, J. J.; Heip, C. H. R. (2007). "Impact of elevated CO
2 on shellfish calcification". Geophysical Research Letters. 34 (7): L07603. Bibcode:2007GeoRL..3407603G. doi:10.1029/2006GL028554. hdl:20.500.11755/a8941c6a-6d0b-43d5-ba0d-157a7aa05668. S2CID 130190489. - Comeau, C.; Gorsky, G.; Jeffree, R.; Teyssié, J.-L.; Gattuso, J.-P. (2009). "Impact of ocean acidification on a key Arctic pelagic mollusc ("Limacina helicina")". Biogeosciences. 6 (9): 1877–1882. Bibcode:2009BGeo....6.1877C. doi:10.5194/bg-6-1877-2009.
- Buitenhuis, E. T.; de Baar, H. J. W.; Veldhuis, M. J. W. (1999). "Photosynthesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species". Journal of Phycology. 35 (5): 949–959. doi:10.1046/j.1529-8817.1999.3550949.x. S2CID 83502030.
- Nimer, N. A.; Merrett, M. J. (1993). "Calcification rate in Emiliania huxleyi Lohmann in response to light, nitrate and availability of inorganic carbon". New Phytologist. 123 (4): 673–677. doi:10.1111/j.1469-8137.1993.tb03776.x.
- Iglesias-Rodriguez, M.D.; Halloran, P.R.; Rickaby, R.E.M.; Hall, I.R.; Colmenero-Hidalgo, E.; Gittins, J.R.; Green, D.R.H.; Tyrrell, T.; Gibbs, S.J.; von Dassow, P.; Rehm, E.; Armbrust, E.V.; Boessenkool, K.P. (2008). "Phytoplankton Calcification in a High-CO2 World". Science. 320 (5874): 336–340. Bibcode:2008Sci...320..336I. doi:10.1126/science.1154122. PMID 18420926. S2CID 206511068.
- Sciandra, A.; Harlay, J.; Lefevre, D.; et al. (2003). "Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation". Marine Ecology Progress Series. 261: 111–112. Bibcode:2003MEPS..261..111S. doi:10.3354/meps261111.
- Langer, G.; Geisen, M.; Baumann, K. H.; et al. (2006). "Species-specific responses of calcifying algae to changing seawater carbonate chemistry" (PDF). Geochemistry, Geophysics, Geosystems. 7 (9): Q09006. Bibcode:2006GGG.....709006L. doi:10.1029/2005GC001227. S2CID 14774230.
- "Acidification Of Oceans May Contribute To Global Declines Of Shellfish, Study By Stony Brook Scientists Concludes" (Press release). School of Marine and Atmospheric Sciences at Stony Brook University. 27 September 2010. Archived from the original on 3 September 2012. Retrieved 4 June 2012.
- Ruttiman, J. (2006). "Sick Seas". Nature. 442 (7106): 978–980. Bibcode:2006Natur.442..978R. doi:10.1038/442978a. PMID 16943816. S2CID 4332965.
- Cohen, A.; Holcomb, M. (2009). "Why Corals Care About Ocean Acidification: Uncovering the Mechanism". Oceanography. 24 (4): 118–127. doi:10.5670/oceanog.2009.102.
- Pérez, F.; Fontela, M.; García-Ibañez, M.; Mercier, H.; Velo, A.; Lherminier, P.; Zunino, P.; de la Paz, M.; Alonso, F.; Guallart, E.; Padín, T. (22 February 2018). "Meridional overturning circulation conveys fast acidification to the deep Atlantic Ocean". Nature. 554 (7693): 515–518. Bibcode:2018Natur.554..515P. doi:10.1038/nature25493. PMID 29433125. S2CID 3497477.
- Mollica, Nathaniel R.; Guo, Weifu; Cohen, Anne L.; Huang, Kuo-Fang; Foster, Gavin L.; Donald, Hannah K.; Solow, Andrew R. (20 February 2018). "Ocean acidification affects coral growth by reducing skeletal density". Proceedings of the National Academy of Sciences. 115 (8): 1754–1759. Bibcode:2018PNAS..115.1754M. doi:10.1073/pnas.1712806115. PMC 5828584. PMID 29378969.
- Albright, R.; Caldeira, L.; Hosfelt, J.; Kwiatkowski, L.; Maclaren, J. K.; Mason, B. M.; Nebuchina, Y.; Ninokawa, A.; Pongratz, J.; Ricke, K. L.; Rivlin, T.; Schneider, K.; Sesboüé, M.; Shamberger, K.; Silverman, J.; Wolfe, K.; Zhu, K.; Caldeira, K. (24 February 2016). "Reversal of ocean acidification enhances net coral reef calcification". Nature. 531 (7594): 362–365. Bibcode:2016Natur.531..362A. doi:10.1038/nature17155. PMID 26909578. S2CID 205247928.
- Albright, R.; Takeshita, T.; Koweek, D. A.; Ninokawa, A.; Wolfe, K.; Rivlin, T.; Nebuchina, Y.; Young, J.; Caldeira, K. (14 March 2018). "Carbon dioxide addition to coral reef waters suppresses net community calcification". Nature. 555 (7697): 516–519. Bibcode:2018Natur.555..516A. doi:10.1038/nature25968. PMID 29539634. S2CID 3935534.
- Hannah L. Wood; John I. Spicer; Stephen Widdicombe (2008). "Ocean acidification may increase calcification rates, but at a cost". Proceedings of the Royal Society B. 275 (1644): 1767–1773. doi:10.1098/rspb.2008.0343. PMC 2587798. PMID 18460426.
- Ducker, James; Falkenberg, Laura J. (2020). "How the Pacific Oyster Responds to Ocean Acidification: Development and Application of a Meta-Analysis Based Adverse Outcome Pathway". Frontiers in Marine Science. 7. doi:10.3389/fmars.2020.597441. ISSN 2296-7745.
- Fabricius, Katharina (2011). "Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations". Nature Climate Change. 1 (3): 165–169. Bibcode:2011NatCC...1..165F. doi:10.1038/nclimate1122. S2CID 85749253.
- Henehan, Michael J.; Ridgwell, Andy; Thomas, Ellen; Zhang, Shuang; Alegret, Laia; Schmidt, Daniela N.; Rae, James W. B.; Witts, James D.; Landman, Neil H.; Greene, Sarah E.; Huber, Brian T. (5 November 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. ISSN 0027-8424. PMC 6842625. PMID 31636204.
- Petrou, Katherina; Nielsen, Daniel (27 August 2019). "Acid oceans are shrinking plankton, fueling faster climate change". phys.org. Retrieved 12 November 2019.
- Hester, K. C.; et al. (2008). "Unanticipated consequences of ocean acidification: A noisier ocean at lower pH". Geophysical Research Letters. 35 (19): L19601. Bibcode:2008GeoRL..3519601H. doi:10.1029/2008GL034913.
- Acid In The Oceans: A Growing Threat To Sea Life by Richard Harris. All Things Considered, 12 August 2009.
- Kwok, Roberta (4 June 2013). "Ocean acidification could make squid develop abnormally". University of Washington. Retrieved 24 August 2013.
- "Swiss marine researcher moving in for the krill". The Australian. 2008. Archived from the original on 11 December 2008. Retrieved 28 September 2008.
- "Ocean Acidification Promotes Disruptive and Harmful Algal Blooms on Our Coasts". 2014.
- Turley, Carol; Gattuso, Jean-Pierre (July 2012). "Future biological and ecosystem impacts of ocean acidification and their socioeconomic-policy implications". Current Opinion in Environmental Sustainability. 4 (3): 278–286. doi:10.1016/j.cosust.2012.05.007.
- Gooding, R.; et al. (2008). "Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm". Proceedings of the National Academy of Sciences. 106 (23): 9316–21. Bibcode:2009PNAS..106.9316G. doi:10.1073/pnas.0811143106. PMC 2695056. PMID 19470464.
- Munday, Philip L. (2009). "Ocean Acidification Impairs Olfactory Discrimination and Homing Ability of a Marine Fish" (PDF). Proceedings of the National Academy of Sciences. 106 (6): 1848–52. Bibcode:2009PNAS..106.1848M. doi:10.1073/pnas.0809996106. PMC 2644126. PMID 19188596.
- Clark, Timothy D.; Raby, Graham D.; Roche, Dominique G.; Binning, Sandra A.; Speers-Roesch, Ben; Jutfelt, Fredrik; Sundin, Josefin (January 2020). "Ocean acidification does not impair the behaviour of coral reef fishes". Nature. 577 (7790): 370–375. Bibcode:2020Natur.577..370C. doi:10.1038/s41586-019-1903-y. ISSN 1476-4687. PMID 31915382. S2CID 210118722.
- Clements, Jeff C.; Sundin, Josefin; Clark, Timothy D.; Jutfelt, Fredrik (3 February 2022). "Meta-analysis reveals an extreme "decline effect" in the impacts of ocean acidification on fish behavior". PLOS Biology. 20 (2): e3001511. doi:10.1371/journal.pbio.3001511. ISSN 1545-7885. PMC 8812914. PMID 35113875.
- Dixson, Danielle L.; Munday, Philip L.; Jones, Geoffrey P. (2010). "Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues". Ecology Letters. 13 (1): 68–75. doi:10.1111/j.1461-0248.2009.01400.x. PMID 19917053.
- Munday, Philip L.; Dixson, Danielle L.; McCormick, Mark I.; Meekan, Mark; Ferrari, Maud C. O.; Chivers, Douglas P. (6 July 2010). "Replenishment of fish populations is threatened by ocean acidification". Proceedings of the National Academy of Sciences. 107 (29): 12930–12934. Bibcode:2010PNAS..10712930M. doi:10.1073/pnas.1004519107. ISSN 0027-8424. PMC 2919925. PMID 20615968.
- Munday, Philip L.; Dixson, Danielle L.; Donelson, Jennifer M.; Jones, Geoffrey P.; Pratchett, Morgan S.; Devitsina, Galina V.; Døving, Kjell B. (10 February 2009). "Ocean acidification impairs olfactory discrimination and homing ability of a marine fish". Proceedings of the National Academy of Sciences. 106 (6): 1848–1852. Bibcode:2009PNAS..106.1848M. doi:10.1073/pnas.0809996106. ISSN 0027-8424. PMC 2644126. PMID 19188596.
- Clements, Jeff C.; Hunt, Heather L. (29 September 2015). "Marine animal behaviour in a high CO2 ocean". Marine Ecology Progress Series. 536: 259–279. Bibcode:2015MEPS..536..259C. doi:10.3354/meps11426. ISSN 0171-8630.
- Clark, Timothy D.; Raby, Graham D.; Roche, Dominique G.; Binning, Sandra A.; Speers-Roesch, Ben; Jutfelt, Fredrik; Sundin, Josefin (January 2020). "Ocean acidification does not impair the behaviour of coral reef fishes". Nature. 577 (7790): 370–375. Bibcode:2020Natur.577..370C. doi:10.1038/s41586-019-1903-y. ISSN 1476-4687. PMID 31915382. S2CID 210118722.
- Chan, F., Barth, J.A., Kroeker, K.J., Lubchenco, J. and Menge, B.A. (2019) "The dynamics and impact of ocean acidification and hypoxia". Oceanography, 32(3): 62–71. doi:10.5670/oceanog.2019.312.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Gewin, V. (2010) "Oceanography: Dead in the water". Nature, 466(7308): 812. doi:10.1038/466812a.
- Kroeker, et al. (June 2013) "Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming." Glob Chang Biol. 19(6): 1884–1896
- Harvey, et al. (April 2013) "Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming." Ecol Evol. 3(4): 1016–1030
- Nagelkerken Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions, PNAS vol. 112 no. 43, 2015
- Bednaršek, N.; Harvey, C.J.; Kaplan, I.C.; Feely, R.A.; Možina, J. (2016). "Pteropods on the edge: Cumulative effects of ocean acidification, warming, and deoxygenation". Progress in Oceanography. 145: 1–24. Bibcode:2016PrOce.145....1B. doi:10.1016/j.pocean.2016.04.002.
- Keeling, Ralph F.; Garcia, Hernan E. (2002). "The change in oceanic O2 inventory associated with recent global warming". Proceedings of the National Academy of Sciences. 99 (12): 7848–7853. Bibcode:2002PNAS...99.7848K. doi:10.1073/pnas.122154899. PMC 122983. PMID 12048249.
- Harvey wt al Ecol Evol. 2013 Apr; 3(4): 1016–1030
- Gruber, Nicolas. "Warming up, turning sour, losing breath: ocean biogeochemistry under global change." Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 369.1943 (2011): 1980–1996.
- Anthony, et al. (May 2011) "Ocean acidification and warming will lower coral reef resilience." Global Change biology, Volume 17, Issue 5, Pages 1798–1808
- Goldenberg, Silvan U, et al. (2017) "Boosted food web productivity through ocean acidification collapses under warming." Global Change Biology.
- Pistevos, Jennifer CA, et al. (2015) "Ocean acidification and global warming impair shark hunting behaviour and growth." Scientific reports 5: 16293.
- Ridgwell, A.; Zondervan, I.; Hargreaves, J. C.; Bijma, J.; Lenton, T. M. (2007). "Assessing the potential long-term increase of oceanic fossil fuel CO2 uptake due to CO2-calcification feedback". Biogeosciences. 4 (4): 481–492. Bibcode:2007BGeo....4..481R. doi:10.5194/bg-4-481-2007.
- Tyrrell, T. (2008). "Calcium carbonate cycling in future oceans and its influence on future climates". Journal of Plankton Research. 30 (2): 141–156. doi:10.1093/plankt/fbm105.
- "Effects of Ocean Acidification on Marine Species & Ecosystems". Report. OCEANA. Retrieved 13 October 2013.
- Lischka, S.; Büdenbender J.; Boxhammer T.; Riebesell U. (15 April 2011). "Impact of ocean acidification and elevated temperatures on early juveniles of the polar shelled pteropod Limacina helicina : mortality, shell degradation, and shell growth" (PDF). Report. Biogeosciences. pp. 919–932. Retrieved 14 November 2013.
- "Comprehensive study of Arctic Ocean acidification". Study. CICERO. Archived from the original on 10 December 2013. Retrieved 14 November 2013.
- "Antarctic marine wildlife is under threat, study finds". BBC Nature. Retrieved 13 October 2013.
- V. J. Fabry; C. Langdon; W. M. Balch; A. G. Dickson; R. A. Feely; B. Hales; D. A. Hutchins; J. A. Kleypas & C. L. Sabine. "Present and Future Impacts of Ocean Acidification on Marine Ecosystems and Biogeochemical Cycles" (PDF). Report of the Ocean Carbon and Biogeochemistry Scoping Workshop on Ocean Acidification Research. Archived from the original (PDF) on 17 November 2010. Retrieved 14 November 2013.
- "Canada's State of the Oceans Report, 2012". Report. Fisheries and Oceans Canada. 2012. Archived from the original on 6 November 2013. Retrieved 21 October 2013.
- Robert J. Foy; Mark Carls; Michael Dalton; Tom Hurst; W. Christopher Long; Dusanka Poljak; André E. Punt; Michael F. Sigler; Robert P. Stone; Katherine M. Swiney (Winter 2013). "CO 2 , pH, and Anticipating a Future under Ocean Acidification" (PDF). ONCORHYNCHUS. Vol. XXXIII, no. 1. Retrieved 14 November 2013.
- "Bering Sea Crab Fishery". Report. Seafood Market Bulletin. November 2005. Archived from the original on 11 December 2013. Retrieved 10 November 2013.
- Snyder, John. "Tourism in the Polar Regions: The Sustainability Challenge" (PDF). Report. UNEP, The International Ecotourism Society. Retrieved 13 October 2013.
- Harvey, Fiona (4 December 2019). "Tackling degraded oceans could mitigate climate crisis - report". The Guardian. ISSN 0261-3077. Retrieved 7 December 2019.
- Clarke & others (2007), Technical Summary, Table TS.2 (p. 9) and Figure TS.10 (p. 20).
- WBGU (2006), Summary for Policymakers, Halting ocean acidification in time, p. 3
- UNFCCC (15 March 2011). "Report of the Conference of the Parties on its sixteenth session, held in Cancun from 29 November to 10 December 2010. Addendum. Part two: Action taken by the Conference of the Parties at its sixteenth session" (PDF). Framework Convention on Climate Change. Geneva, Switzerland: United Nations. p. 3, paragraph 4. Document available in UN languages and text format.
- UNEP (2010), Ch 2: Which emission pathways are consistent with a 2 °C or 1.5 °C temperature limit?, pp. 28–29.
- Good & others (2010), Executive Summary.
- "Carbon Dioxide Emissions and Ocean Acidification; TSCA Section 21 Petition; Reasons for Agency Response". Environmental Protection Agency (EPA). 7 October 2015.
- Center for Biological Diversity; Donn J. Viviani. "TSCA Section 21 Petition Requesting EPA to Regulate Anthropogenic Emissions Carbon Dioxide" (PDF). US EPA.
- "The President's Climate Action Plan" (PDF). Retrieved 27 June 2017.
- Dan Merica (28 March 2017). "Trump dramatically changes US approach to climate change". CNN Politics. CNN.
- Shear, Michael D. (1 June 2017). "Trump Will Withdraw U.S. From Paris Climate Agreement". The New York Times.
- "US opts out of G7 pledge stating Paris climate accord is 'irreversible'". The Guardian. Associated Press, Bologna. 12 June 2017.
- "Federal Register :: Request Access".
- https://www.epa.gov/system/files/documents/2022-07/GHG_TSCA%20Section%2021_AX-22-000-4960.pdf
- "How Can Trees Help the Seas?". The Nature Conservancy. Retrieved 3 September 2021.
- UK Royal Society (2009), Summary, pp. ix–xii.
- US NRC (2011), Ch 5: Key Elements of America's Climate Choices, Box 5.1: Geoengineering, pp. 52–53.
- Trujillo, Alan (2011). Essentials of Oceanography. Pearson Education, Inc. p. 157. ISBN 9780321668127.
- Cao, L.; Caldeira, K. (2010). "Can ocean iron fertilization mitigate ocean acidification?". Climatic Change. 99 (1–2): 303–311. Bibcode:2010ClCh...99..303C. doi:10.1007/s10584-010-9799-4. S2CID 153613458.
- UK Royal Society (2009), Ch 2: Carbon dioxide removal techniques,Sec 2.3.1 Ocean fertilisation methods, pp. 16–19.
- UK Royal Society (2009), Ch 2: Carbon dioxide removal techniques, Sec 2.3.1 Ocean fertilisation methods, Table 2.8, p. 18.
- Ritchie, Roser, Mispy, Ortiz-Ospina. "SDG 14 - Measuring progress towards the Sustainable Development Goals." SDG-Tracker.org, website (2018).
- United Nations (2017) Resolution adopted by the General Assembly on 6 July 2017, Work of the Statistical Commission pertaining to the 2030 Agenda for Sustainable Development (A/RES/71/313)
- "Goal 14: Sustainable Development Knowledge Platform". sustainabledevelopment.un.org. Retrieved 5 September 2020.
- Zeebe, R.E. (2012). "History of Seawater Carbonate Chemistry, Atmospheric CO2, and Ocean Acidification". Annual Review of Earth and Planetary Sciences. 40 (1): 141–165. Bibcode:2012AREPS..40..141Z. doi:10.1146/annurev-earth-042711-105521. S2CID 18682623.
- Henehan, Michael J.; Ridgwell, Andy; Thomas, Ellen; Zhang, Shuang; Alegret, Laia; Schmidt, Daniela N.; Rae, James W. B.; Witts, James D.; Landman, Neil H.; Greene, Sarah E.; Huber, Brian T. (17 October 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. ISSN 0027-8424. PMC 6842625. PMID 31636204.
- Carrington, Damian (21 October 2019). "Ocean acidification can cause mass extinctions, fossils reveal". The Guardian. ISSN 0261-3077. Retrieved 22 October 2019.
- Zachos, J.C.; Röhl, U.; Schellenberg, S.A.; Sluijs, A.; Hodell, D.A.; Kelly, D.C.; Thomas, E.; Nicolo, M.; Raffi, I.; Lourens, L. J.; McCarren, H.; Kroon, D. (2005). "Rapid acidification of the ocean during the Paleocene-Eocene thermal maximum". Science. 308 (5728): 1611–1615. Bibcode:2005Sci...308.1611Z. doi:10.1126/science.1109004. hdl:1874/385806. PMID 15947184. S2CID 26909706.
- Beerling, D. J.; Berner, R. A. (September 2002). "Biogeochemical constraints on the Triassic-Jurassic boundary carbon cycle event: TR-J BOUNDARY C-CYCLE DYNAMICS". Global Biogeochemical Cycles. 16 (3): 10–1–10–13. Bibcode:2002GBioC..16.1036B. doi:10.1029/2001GB001637. S2CID 53590993.
- Bond, David P.G.; Wignall, Paul B. (2014), "Large igneous provinces and mass extinctions: An update", Volcanism, Impacts, and Mass Extinctions: Causes and Effects, Geological Society of America, pp. 29–55, doi:10.1130/2014.2505(02), ISBN 978-0-8137-2505-5, retrieved 4 May 2020
- Hallam, A. (1997). Mass extinctions and their aftermath. Oxford University Press. ISBN 0-19-854917-2. OCLC 37141126.
- Hautmann, M. (2004). "Effect of end-Triassic CO2 maximum on carbonate sedimentation and marine mass extinction". Facies. 50 (2). doi:10.1007/s10347-004-0020-y. ISSN 0172-9179. S2CID 130658467.
- Hautmann, Michael; Benton, Michael J.; Tomašových, Adam (1 July 2008). "Catastrophic ocean acidification at the Triassic-Jurassic boundary". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen. 249 (1): 119–127. doi:10.1127/0077-7749/2008/0249-0119.
- Greene, Sarah E.; Martindale, Rowan C.; Ritterbush, Kathleen A.; Bottjer, David J.; Corsetti, Frank A.; Berelson, William M. (June 2012). "Recognising ocean acidification in deep time: An evaluation of the evidence for acidification across the Triassic-Jurassic boundary". Earth-Science Reviews. 113 (1–2): 72–93. Bibcode:2012ESRv..113...72G. doi:10.1016/j.earscirev.2012.03.009.
- Blackburn, T. J.; Olsen, P. E.; Bowring, S. A.; McLean, N. M.; Kent, D. V.; Puffer, J.; McHone, G.; Rasbury, E. T.; Et-Touhami, M. (21 March 2013). "Zircon U-Pb Geochronology Links the End-Triassic Extinction with the Central Atlantic Magmatic Province". Science. 340 (6135): 941–945. Bibcode:2013Sci...340..941B. doi:10.1126/science.1234204. ISSN 0036-8075. PMID 23519213. S2CID 15895416.
- Lindström, Sofie; van de Schootbrugge, Bas; Hansen, Katrine H.; Pedersen, Gunver K.; Alsen, Peter; Thibault, Nicolas; Dybkjær, Karen; Bjerrum, Christian J.; Nielsen, Lars Henrik (July 2017). "A new correlation of Triassic–Jurassic boundary successions in NW Europe, Nevada and Peru, and the Central Atlantic Magmatic Province: A time-line for the end-Triassic mass extinction". Palaeogeography, Palaeoclimatology, Palaeoecology. 478: 80–102. Bibcode:2017PPP...478...80L. doi:10.1016/j.palaeo.2016.12.025. hdl:1874/351998. S2CID 133353132.
- Hautmann, M.; Stiller, F.; Huawei, C.; Jingeng, S. (1 October 2008). "Extinction-Recovery Pattern of Level-Bottom Faunas Across the Triassic-Jurassic Boundary in Tibet: Implications for Potential Killing Mechanisms". PALAIOS. 23 (10): 711–718. Bibcode:2008Palai..23..711H. doi:10.2110/palo.2008.p08-005r. ISSN 0883-1351. S2CID 42675849.
- Hautmann, Michael (15 August 2012), "Extinction: End-Triassic Mass Extinction", eLS, John Wiley & Sons, pp. a0001655.pub3, doi:10.1002/9780470015902.a0001655.pub3, ISBN 978-0-470-01617-6, S2CID 130434497
- Riebesell, Ulf; Zondervan, Ingrid; Rost, Björn; Tortell, Philippe D.; Zeebe, Richard E.; Morel, François M. M. (September 2000). "Reduced calcification of marine plankton in response to increased atmospheric CO2" (PDF). Nature. 407 (6802): 364–367. Bibcode:2000Natur.407..364R. doi:10.1038/35030078. ISSN 0028-0836. PMID 11014189. S2CID 4426501.
- Fine, M.; Tchernov, D. (30 March 2007). "Scleractinian Coral Species Survive and Recover from Decalcification". Science. 315 (5820): 1811. Bibcode:2007Sci...315.1811F. doi:10.1126/science.1137094. ISSN 0036-8075. PMID 17395821. S2CID 28535145.
- Payne, J. L.; Lehrmann, D. J.; Follett, D.; Seibel, M.; Kump, L. R.; Riccardi, A.; Altiner, D.; Sano, H.; Wei, J. (1 July 2007). "Erosional truncation of uppermost Permian shallow-marine carbonates and implications for Permian-Triassic boundary events". Geological Society of America Bulletin. 119 (7–8): 771–784. Bibcode:2007GSAB..119..771P. doi:10.1130/B26091.1. hdl:11511/35436. ISSN 0016-7606.
- Clarkson, M. O.; Kasemann, S. A.; Wood, R. A.; Lenton, T. M.; Daines, S. J.; Richoz, S.; Ohnemueller, F.; Meixner, A.; Poulton, S. W.; Tipper, E. T. (10 April 2015). "Ocean acidification and the Permo-Triassic mass extinction" (PDF). Science. 348 (6231): 229–232. Bibcode:2015Sci...348..229C. doi:10.1126/science.aaa0193. ISSN 0036-8075. PMID 25859043. S2CID 28891777.
- Henehan, Michael J.; Ridgwell, Andy; Thomas, Ellen; Zhang, Shuang; Alegret, Laia; Schmidt, Daniela N.; Rae, James W. B.; Witts, James D.; Landman, Neil H.; Greene, Sarah E.; Huber, Brian T. (5 November 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. ISSN 0027-8424. PMC 6842625. PMID 31636204.
Sources
- Clarke, L.; Edmonds, J.; Jacoby, H.; Pitcher, H.; Reilly, J.; Richels, R. (July 2007). "Scenarios of Greenhouse Gas Emissions and Atmospheric Concentrations. Sub-report 2.1A". In U.S. Climate Change Science Program and the Subcommittee on Global Change Research (ed.). Synthesis and Assessment Product 2.1. Washington, DC., USA: Department of Energy, Office of Biological & Environmental Research. Archived from the original (PDF) on 16 June 2013.
- Good, P.; Gosling, S. N.; Bernie, D.; Caesar1, J.; Warren, R.; Arnell, N. W.; Lowe, J. A. (2010). An updated review of developments in climate science research since IPCC Fourth Assessment Report (PDF) (Report). London, UK: AVOID Consortium. Report website.
- UK Royal Society (September 2009). Geoengineering the climate: science, governance and uncertainty (PDF). London: UK Royal Society. ISBN 978-0-85403-773-5, RS Policy document 10/09.
{{cite book}}: CS1 maint: postscript (link) Report website. - UNEP (November 2010). The Emissions Gap Report: Are the Copenhagen Accord pledges sufficient to limit global warming to 2°C or 1.5°C? A preliminary assessment. Nairobi, Kenya: United Nations Environment Programme (UNEP). ISBN 978-92-807-3134-7.
- US National Research Council (US NRC) (2011). America's Climate Choices. Washington, DC, USA: National Academies Press. doi:10.17226/12781. ISBN 978-0-309-14585-5.
- WBGU (2006). Special Report: The Future Oceans – Warming Up, Rising High, Turning Sour (PDF). Berlin, Germany: German Advisory Council on Global Change (WBGU). ISBN 978-3-936191-14-1. Report website.