Asthma
| Asthma | |
|---|---|
 | |
| Specialty | Pulmonology |
| Symptoms | Recurring episodes of wheezing, coughing, chest tightness, shortness of breath[1] |
| Usual onset | Typically in childhood / before age 25 years[1] |
| Duration | Long term[2] |
| Causes | Genetic and environmental factors[1] |
| Risk factors | Air pollution, allergens (viral infection, dust, smoke, animal fur, perfume), some medicines[3][4] |
| Diagnostic method | Based on symptoms, response to therapy, spirometry[2] |
| Treatment | Avoiding triggers, inhaled corticosteroids, salbutamol[3] |
| Frequency | 262 million (2019)[3] |
| Deaths | 455,000 (2019)[3] |
Asthma is a common long-term inflammatory disease of the airways of the lungs.[1] It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms; classically wheezing, coughing, shortness of breath, and chest tightness.[2][5] These may occur a few times a day or a few times per week.[6] Symptoms may disturb sleep or worsen with exercise.[2] Asthma may also present with tiredness, recurrent colds, or poor fitness.[2]
Asthma is thought to be caused by a combination of genetic and environmental factors.[1] Environmental factors include exposure to air pollution and allergens.[3] Other potential triggers include medications such as aspirin and other NSAIDs, beta blockers, and ACE inhibitors.[4] Diagnosis is generally based on the pattern of symptoms, response to therapy over time, and spirometry lung function testing.[2] Asthma is classified according to the frequency of symptoms, forced expiratory volume in one second (FEV1), and peak expiratory flow rate.[2] It has traditionally been classified as atopic and non-atopic; thought this is an over simplification.[1]
There is no cure.[3] Symptoms can be prevented by avoiding triggers, such as allergens and irritants, and controlled by the use of inhaled corticosteroids.[3] Long-acting beta agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and antileukotriene agents may be used in addition to inhaled corticosteroids if symptoms remain uncontrolled.[1] Treatment of rapidly worsening symptoms is usually with an inhaled short-acting beta-2 agonist such as salbutamol and corticosteroids taken by mouth.[7] In very severe cases, intravenous corticosteroids, magnesium sulfate, and hospitalization may be required.[8]
In 2019, 262 million people globally had asthma, up from 183 million in 1990.[3][9] It caused about 455,000 deaths in 2019, most of which occurred in the developing world.[3] Asthma typically begins in childhood, more frequently before age 25 years, but the onset can be at any age.[1] Boys are affected more frequently than girls and women more than men.[1] Rates doubled over the second half of the 20th century, with higher rates in industrialized countries.[1] There appears to be lower rates in people growing up in farming environments.[1] Asthma was recognized as early as Ancient Egypt.[10] The word "asthma" is from the Greek ἅσθμα, ásthma, which means "panting".[11][12]
Signs and symptoms
Asthma is characterized by recurrent episodes of wheezing, shortness of breath, chest tightness, and coughing.[2] Sputum may be produced from the lung by coughing but is often hard to bring up.[13] During recovery from an asthma attack (exacerbation), it may appear pus-like due to high levels of white blood cells called eosinophils.[14] Symptoms are usually worse at night and in the early morning or in response to exercise or cold air.[15] Some people with asthma rarely experience symptoms, usually in response to triggers, whereas others may react frequently and readily and experience persistent symptoms.[16]
Associated conditions
A number of other health conditions occur more frequently in people with asthma, including gastro-esophageal reflux disease (GERD), rhinosinusitis, and obstructive sleep apnea.[17] Psychological disorders are also more common,[18] with anxiety disorders occurring in between 16–52% and mood disorders in 14–41%.[19] It is not known whether asthma causes psychological problems or psychological problems lead to asthma.[20] Those with asthma, especially if it is poorly controlled, are at increased risk for radiocontrast reactions.[21]
Cavities occur more often in people with asthma.[22] This may be related to the effect of beta 2 agonists decreasing saliva.[23] These medications may also increase the risk of dental erosions.[23]
Causes
Asthma is caused by a combination of complex and incompletely understood environmental and genetic interactions.[1] These influence both its severity and its responsiveness to treatment.[24] It is believed that the recent increased rates of asthma are due to changing epigenetics (heritable factors other than those related to the DNA sequence) and a changing living environment.[25] Asthma that starts before the age of 12 years old is more likely due to genetic influence, while onset after age 12 is more likely due to environmental influence.[26]
Environmental
Many environmental factors have been associated with asthma's development and exacerbation, including, allergens, air pollution, and other environmental chemicals.[27] Smoking during pregnancy and after delivery is associated with a greater risk of asthma-like symptoms.[28] Low air quality from environmental factors such as traffic pollution or high ozone levels[29] has been associated with both asthma development and increased asthma severity.[30] Over half of cases in children in the United States occur in areas when air quality is below the EPA standards.[31] Low air quality is more common in low-income and minority communities.[32]
Exposure to indoor volatile organic compounds may be a trigger for asthma; formaldehyde exposure, for example, has a positive association.[33] Phthalates in certain types of PVC are associated with asthma in both children and adults.[34][35] While exposure to pesticides is linked to the development of asthma, a cause and effect relationship has yet to be established.[36][37]
The majority of the evidence does not support a causal role between acetaminophen (paracetamol) or antibiotic use and asthma.[38][39] A 2014 systematic review found that the association between acetaminophen use and asthma disappeared when respiratory infections were taken into account.[40] Acetaminophen use by a mother during pregnancy is also associated with an increased risk of the child developing asthma.[41] Maternal psychological stress during pregnancy is a risk factor for the child to develop asthma.[42]
Asthma is associated with exposure to indoor allergens.[43] Common indoor allergens include dust mites, cockroaches, animal dander (fragments of fur or feathers), and mold.[44][45] Efforts to decrease dust mites have been found to be ineffective on symptoms in sensitized subjects.[46][47] Weak evidence suggests that efforts to decrease mold by repairing buildings may help improve asthma symptoms in adults.[48] Certain viral respiratory infections, such as respiratory syncytial virus and rhinovirus,[11] may increase the risk of developing asthma when acquired as young children.[49] Certain other infections, however, may decrease the risk.[11]
Hygiene hypothesis
The hygiene hypothesis attempts to explain the increased rates of asthma worldwide as a direct and unintended result of reduced exposure, during childhood, to non-pathogenic bacteria and viruses.[50][51] It has been proposed that the reduced exposure to bacteria and viruses is due, in part, to increased cleanliness and decreased family size in modern societies.[52] Exposure to bacterial endotoxin in early childhood may prevent the development of asthma, but exposure at an older age may provoke bronchoconstriction.[53] Evidence supporting the hygiene hypothesis includes lower rates of asthma on farms and in households with pets.[52]
Use of antibiotics in early life has been linked to the development of asthma.[54] Also, delivery via caesarean section is associated with an increased risk (estimated at 20–80%) of asthma – this increased risk is attributed to the lack of healthy bacterial colonization that the newborn would have acquired from passage through the birth canal.[55][56] There is a link between asthma and the degree of affluence which may be related to the hygiene hypothesis as less affluent individuals often have more exposure to bacteria and viruses.[57]
Genetic
| Endotoxin levels | CC genotype | TT genotype |
|---|---|---|
| High exposure | Low risk | High risk |
| Low exposure | High risk | Low risk |
Family history is a risk factor for asthma, with many different genes being implicated.[59] If one identical twin is affected, the probability of the other having the disease is approximately 25%.[59] By the end of 2005, 25 genes had been associated with asthma in six or more separate populations, including GSTM1, IL10, CTLA-4, SPINK5, LTC4S, IL4R and ADAM33, among others.[60] Many of these genes are related to the immune system or modulating inflammation. Even among this list of genes supported by highly replicated studies, results have not been consistent among all populations tested.[60] In 2006 over 100 genes were associated with asthma in one genetic association study alone;[60] more continue to be found.[61]
Some genetic variants may only cause asthma when they are combined with specific environmental exposures.[58] An example is a specific single nucleotide polymorphism in the CD14 region and exposure to endotoxin (a bacterial product). Endotoxin exposure can come from several environmental sources including tobacco smoke, dogs, and farms. Risk for asthma, then, is determined by both a person's genetics and the level of endotoxin exposure.[62]
Medical conditions
A triad of atopic eczema, allergic rhinitis and asthma is called atopy.[63] The strongest risk factor for developing asthma is a history of atopic disease;[1] with asthma occurring at a much greater rate in those who have either eczema or hay fever.[64] Asthma has been associated with eosinophilic granulomatosis with polyangiitis (formerly known as Churg–Strauss syndrome), an autoimmune disease and vasculitis.[65] Individuals with certain types of urticaria may also experience symptoms of asthma.[63]
There is a correlation between obesity and the risk of asthma with both having increased in recent years.[66][67] Several factors may be at play including decreased respiratory function due to a buildup of fat and the fact that adipose tissue leads to a pro-inflammatory state.[68]
Beta blocker medications such as propranolol can trigger asthma in those who are susceptible.[69] Cardioselective beta-blockers, however, appear safe in those with mild or moderate disease.[70][71] Other medications that can cause problems in asthmatics are angiotensin-converting enzyme inhibitors, aspirin, and NSAIDs.[4] Use of acid suppressing medication (proton pump inhibitors and H2 blockers) during pregnancy is associated with an increased risk of asthma in the child.[72]
Exacerbation
Some individuals will have stable asthma for weeks or months and then suddenly develop an episode of acute asthma. Different individuals react to various factors in different ways.[73] Most individuals can develop severe exacerbation from a number of triggering agents.[73]
Home factors that can lead to exacerbation of asthma include dust, animal dander (especially cat and dog hair), cockroach allergens and mold.[73][74] Perfumes are a common cause of acute attacks in women and children. Both viral and bacterial infections of the upper respiratory tract can worsen the disease.[73] Psychological stress may worsen symptoms – it is thought that stress alters the immune system and thus increases the airway inflammatory response to allergens and irritants.[30][75]
Asthma exacerbations in school‐aged children peak in autumn, shortly after children return to school. This might reflect a combination of factors, including poor treatment adherence, increased allergen and viral exposure, and altered immune tolerance. There is limited evidence to guide possible approaches to reducing autumn exacerbations, but while costly, seasonal omalizumab treatment from four to six weeks before school return may reduce autumn asthma exacerbations.[76]
Pathophysiology
_constriction-animated.gif)
Asthma is the result of chronic inflammation of the conducting zone of the airways (most especially the bronchi and bronchioles), which subsequently results in increased contractability of the surrounding smooth muscles. This among other factors leads to bouts of narrowing of the airway and the classic symptoms of wheezing. The narrowing is typically reversible with or without treatment. Occasionally the airways themselves change.[15] Typical changes in the airways include an increase in eosinophils and thickening of the lamina reticularis. Chronically the airways' smooth muscle may increase in size along with an increase in the numbers of mucous glands. Other cell types involved include: T lymphocytes, macrophages, and neutrophils. There may also be involvement of other components of the immune system including: cytokines, chemokines, histamine, and leukotrienes among others.[11]
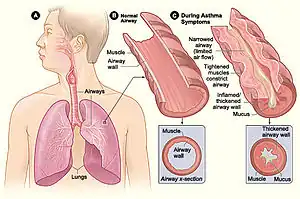 Figure A shows the location of the lungs and airways in the body. Figure B shows a cross-section of a normal airway. Figure C shows a cross-section of an airway during asthma symptoms.
Figure A shows the location of the lungs and airways in the body. Figure B shows a cross-section of a normal airway. Figure C shows a cross-section of an airway during asthma symptoms.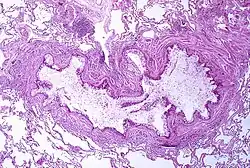 Obstruction of the lumen of a bronchiole by mucoid exudate, goblet cell metaplasia, and epithelial basement membrane thickening in a person with asthma.
Obstruction of the lumen of a bronchiole by mucoid exudate, goblet cell metaplasia, and epithelial basement membrane thickening in a person with asthma.
Diagnosis
While asthma is a well-recognized condition, there is not one universal agreed upon definition.[11] It is defined by the Global Initiative for Asthma as "a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyper-responsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing particularly at night or in the early morning. These episodes are usually associated with widespread but variable airflow obstruction within the lung that is often reversible either spontaneously or with treatment".[15]
There is currently no precise test for the diagnosis, which is typically based on the pattern of symptoms and response to therapy over time.[78][11] A diagnosis of asthma should be suspected if there is a history of recurrent wheezing, coughing or difficulty breathing and these symptoms occur or worsen due to exercise, viral infections, allergens or air pollution.[79] Spirometry is then used to confirm the diagnosis.[79] In children under the age of six the diagnosis is more difficult as they are too young for spirometry.[80]
Spirometry
Spirometry is recommended to aid in diagnosis and management.[81][82] It is the single best test for asthma. If the FEV1 measured by this technique improves more than 12% and increases by at least 200 milliliters following administration of a bronchodilator such as salbutamol, this is supportive of the diagnosis. It however may be normal in those with a history of mild asthma, not currently acting up.[11] As caffeine is a bronchodilator in people with asthma, the use of caffeine before a lung function test may interfere with the results.[83] Single-breath diffusing capacity can help differentiate asthma from COPD.[11] It is reasonable to perform spirometry every one or two years to follow how well a person's asthma is controlled.[84]
Others
The methacholine challenge involves the inhalation of increasing concentrations of a substance that causes airway narrowing in those predisposed. If negative it means that a person does not have asthma; if positive, however, it is not specific for the disease.[11]
Other supportive evidence includes: a ≥20% difference in peak expiratory flow rate on at least three days in a week for at least two weeks, a ≥20% improvement of peak flow following treatment with either salbutamol, inhaled corticosteroids or prednisone, or a ≥20% decrease in peak flow following exposure to a trigger.[85] Testing peak expiratory flow is more variable than spirometry, however, and thus not recommended for routine diagnosis. It may be useful for daily self-monitoring in those with moderate to severe disease and for checking the effectiveness of new medications. It may also be helpful in guiding treatment in those with acute exacerbations.[86]
Classification
| Severity | Symptom frequency | Night-time symptoms | %FEV1 of predicted | FEV1 variability | SABA use |
|---|---|---|---|---|---|
| Intermittent | ≤2/week | ≤2/month | ≥80% | <20% | ≤2 days/week |
| Mild persistent | >2/week | 3–4/month | ≥80% | 20–30% | >2 days/week |
| Moderate persistent | Daily | >1/week | 60–80% | >30% | daily |
| Severe persistent | Continuously | Frequent (7/week) | <60% | >30% | ≥twice/day |
Asthma is clinically classified according to the frequency of symptoms, forced expiratory volume in one second (FEV1), and peak expiratory flow rate.[87] Asthma has traditionally been classified as atopic (extrinsic) or non-atopic (intrinsic), based on whether symptoms are precipitated by allergens (atopic) or not (non-atopic); now thought to be an oversimplification.[1] While asthma is classified based on severity, at the moment there is no clear method for classifying different subgroups of asthma beyond this system.[88] Finding ways to identify subgroups that respond well to different types of treatments is a current critical goal of asthma research.[88]
Although asthma is a chronic obstructive condition, it is not considered as a part of chronic obstructive pulmonary disease, as this term refers specifically to combinations of disease that are irreversible such as bronchiectasis and emphysema.[89] Unlike these diseases, the airway obstruction in asthma is usually reversible; however, if left untreated, the chronic inflammation from asthma can lead the lungs to become irreversibly obstructed due to airway remodeling.[90] In contrast to emphysema, asthma affects the bronchi, not the alveoli.[91]
Asthma exacerbation
| Near-fatal | High PaCO2, or requiring mechanical ventilation, or both | |
|---|---|---|
| Life-threatening (any one of) | ||
| Clinical signs | Measurements | |
| Altered level of consciousness | Peak flow < 33% | |
| Exhaustion | Oxygen saturation < 92% | |
| Arrhythmia | PaO2 < 8 kPa | |
| Low blood pressure | "Normal" PaCO2 | |
| Cyanosis | ||
| Silent chest | ||
| Poor respiratory effort | ||
| Acute severe (any one of) | ||
| Peak flow 33–50% | ||
| Respiratory rate ≥ 25 breaths per minute | ||
| Heart rate ≥ 110 beats per minute | ||
| Unable to complete sentences in one breath | ||
| Moderate | Worsening symptoms | |
| Peak flow 50–80% best or predicted | ||
| No features of acute severe asthma | ||
An acute asthma exacerbation is commonly referred to as an asthma attack. The classic symptoms are shortness of breath, wheezing, and chest tightness.[11] The wheezing is most often when breathing out.[93] While these are the primary symptoms of asthma,[94] some people present primarily with coughing, and in severe cases, air motion may be significantly impaired such that no wheezing is heard.[92] In children, chest pain is often present.[95]
Signs occurring during an asthma attack include the use of accessory muscles of respiration (sternocleidomastoid and scalene muscles of the neck), there may be a paradoxical pulse (a pulse that is weaker during inhalation and stronger during exhalation), and over-inflation of the chest.[96] A blue color of the skin and nails may occur from lack of oxygen.[97]
In a mild exacerbation the peak expiratory flow rate (PEFR) is ≥200 L/min, or ≥50% of the predicted best.[98] Moderate is defined as between 80 and 200 L/min, or 25% and 50% of the predicted best, while severe is defined as ≤ 80 L/min, or ≤25% of the predicted best.[98]
Acute severe asthma, previously known as status asthmaticus, is an acute exacerbation of asthma that does not respond to standard treatments of bronchodilators and corticosteroids.[99] Half of cases are due to infections with others caused by allergen, air pollution, or insufficient or inappropriate medication use.[99]
Brittle asthma is a kind of asthma distinguishable by recurrent, severe attacks.[92] Type 1 brittle asthma is a disease with wide peak flow variability, despite intense medication. Type 2 brittle asthma is background well-controlled asthma with sudden severe exacerbations.[92]
Exercise-induced
Exercise can trigger bronchoconstriction both in people with or without asthma.[100] It occurs in most people with asthma and up to 20% of people without asthma.[100] Exercise-induced bronchoconstriction is common in professional athletes. The highest rates are among cyclists (up to 45%), swimmers, and cross-country skiers.[101] While it may occur with any weather conditions, it is more common when it is dry and cold.[102] Inhaled beta2-agonists do not appear to improve athletic performance among those without asthma,[103] however, oral doses may improve endurance and strength.[104][105]
Occupational
Asthma as a result of (or worsened by) workplace exposures is a commonly reported occupational disease.[106] Many cases, however, are not reported or recognized as such.[107][108] It is estimated that 5–25% of asthma cases in adults are work-related. A few hundred different agents have been implicated, with the most common being: isocyanates, grain and wood dust, colophony, soldering flux, latex, animals, and aldehydes. The employment associated with the highest risk of problems include: those who spray paint, bakers and those who process food, nurses, chemical workers, those who work with animals, welders, hairdressers and timber workers.[106]
Aspirin-induced asthma
Aspirin-exacerbated respiratory disease (AERD), also known as aspirin-induced asthma, affects up to 9% of asthmatics.[109] AERD consists of asthma, nasal polyps, sinus disease, and respiratory reactions to aspirin and other NSAID medications (such as ibuprofen and naproxen).[110] People often also develop loss of smell and most experience respiratory reactions to alcohol.[111]
Alcohol-induced asthma
Alcohol may worsen asthmatic symptoms in up to a third of people.[112] This may be even more common in some ethnic groups such as the Japanese and those with aspirin-induced asthma.[112] Other studies have found improvement in asthmatic symptoms from alcohol.[112]
Non-atopic asthma
Non-atopic asthma, also known as intrinsic or non-allergic, makes up between 10 and 33% of cases. There is negative skin test to common inhalant allergens and normal serum concentrations of IgE. Often it starts later in life, and women are more commonly affected than men. Usual treatments may not work as well.[113]
Differential diagnosis
Many other conditions can cause symptoms similar to those of asthma. In children, other upper airway diseases such as allergic rhinitis and sinusitis should be considered as well as other causes of airway obstruction including foreign body aspiration, tracheal stenosis, laryngotracheomalacia, vascular rings, enlarged lymph nodes or neck masses.[114] Bronchiolitis and other viral infections may also produce wheezing.[115] In adults, COPD, congestive heart failure, airway masses, as well as drug-induced coughing due to ACE inhibitors should be considered. In both populations vocal cord dysfunction may present similarly.[114]
Chronic obstructive pulmonary disease can coexist with asthma and can occur as a complication of chronic asthma. After the age of 65, most people with obstructive airway disease will have asthma and COPD. In this setting, COPD can be differentiated by increased airway neutrophils, abnormally increased wall thickness, and increased smooth muscle in the bronchi. However, this level of investigation is not performed due to COPD and asthma sharing similar principles of management: corticosteroids, long-acting beta-agonists, and smoking cessation.[116] It closely resembles asthma in symptoms, is correlated with more exposure to cigarette smoke, an older age, less symptom reversibility after bronchodilator administration, and decreased likelihood of family history of atopy.[117][118]
Prevention
The evidence for the effectiveness of measures to prevent the development of asthma is weak.[119] The World Health Organization recommends decreasing risk factors such as tobacco smoke, air pollution, chemical irritants including perfume, and the number of lower respiratory infections.[120][121] Other efforts that show promise include: limiting smoke exposure in utero, breastfeeding, and increased exposure to daycare or large families, but none are well supported enough to be recommended for this indication.[119]
Early pet exposure may be useful.[122] Results from exposure to pets at other times are inconclusive[123] and it is only recommended that pets be removed from the home if a person has allergic symptoms to said pet.[124]
Dietary restrictions during pregnancy or when breast feeding have not been found to be effective at preventing asthma in children and are not recommended.[124] Reducing or eliminating compounds known to sensitive people from the work place may be effective.[106] It is not clear if annual influenza vaccinations affects the risk of exacerbations.[125] Immunization, however, is recommended by the World Health Organization.[126] Smoking bans are effective in decreasing exacerbations of asthma.[127]
Management
While there is no cure for asthma, symptoms can typically be improved.[128] A specific, customized plan for proactively monitoring and managing symptoms should be created. This plan should include the reduction of exposure to allergens, testing to assess the severity of symptoms, and the usage of and adjustments to medications.[129] The treatment plan should be written down and advise adjustments to treatment according to changes in symptoms.[130]
The most effective treatment for asthma is identifying triggers, such as cigarette smoke, pets, or aspirin, and eliminating exposure to them. If trigger avoidance is insufficient, the use of medication is recommended. Pharmaceutical drugs are selected based on, among other things, the severity of illness and the frequency of symptoms. Specific medications for asthma are broadly classified into fast-acting and long-acting categories.[131][132]
Bronchodilators are recommended for short-term relief of symptoms. In those with occasional attacks, no other medication is needed. If mild persistent disease is present (more than two attacks a week), low-dose inhaled corticosteroids or alternatively, a leukotriene antagonist or a mast cell stabilizer by mouth is recommended. For those who have daily attacks, a higher dose of inhaled corticosteroids is used. In a moderate or severe exacerbation, corticosteroids by mouth are added to these treatments.[7]
People with asthma have higher rates of anxiety, psychological stress, and depression.[133][134] This is associated with poorer asthma control.[133] Cognitive behavioral therapy may improve quality of life, asthma control, and anxiety levels in people with asthma.[133]
Improving people's knowledge about asthma and using a written action plan has been identified as an important component of managing asthma.[135] Providing educational sessions that include information specific to a person's culture is likely effective.[136] More research is necessary to determine if increasing preparedness and knowledge of asthma among school staff and families using home-based and school interventions results in long term improvements in safety for children with asthma.[137][138][139] School-based asthma self-management interventions, which attempt to improve knowledge of asthma, its triggers and the importance of regular practitioner review, may reduce hospital admissions and emergency department visits. These interventions may also reduce the number of days children experience asthma symptoms and may lead to small improvements in asthma-related quality of life.[140] More research is necessary to determine if shared-decision-making is helpful for managing adults with asthma[141] or if a personalized asthma action plan is effective and necessary.[142] Some people with asthma use pulse oximeters to monitor their own blood oxygen levels during an asthma attack. However, there is no evidence regarding the use in these instances.[143]
Lifestyle modification
Avoidance of triggers is a key component of improving control and preventing attacks. The most common triggers include allergens, smoke (from tobacco or other sources), air pollution, non selective beta-blockers, and sulfite-containing foods.[144][145] Cigarette smoking and second-hand smoke (passive smoke) may reduce the effectiveness of medications such as corticosteroids.[146] Laws that limit smoking decrease the number of people hospitalized for asthma.[127] Dust mite control measures, including air filtration, chemicals to kill mites, vacuuming, mattress covers and others methods had no effect on asthma symptoms.[46] There is insufficient evidence to suggest that dehumidifiers are helpful for controlling asthma.[147]
Overall, exercise is beneficial in people with stable asthma.[148] Yoga could provide small improvements in quality of life and symptoms in people with asthma.[149] More research is necessary to determine how effective weight loss is on improving quality of life, the usage of health care services, and adverse effects for people of all ages with asthma.[150][151]
Medications
Medications used to treat asthma are divided into two general classes: quick-relief medications used to treat acute symptoms; and long-term control medications used to prevent further exacerbation.[131] Antibiotics are generally not needed for sudden worsening of symptoms or for treating asthma at any time.[152][153]
Fast–acting

- Short-acting beta2-adrenoceptor agonists (SABA), such as salbutamol (albuterol USAN) are the first line treatment for asthma symptoms.[7] They are recommended before exercise in those with exercise induced symptoms.[154]
- Anticholinergic medications, such as ipratropium, provide additional benefit when used in combination with SABA in those with moderate or severe symptoms and may prevent hospitalizations.[7][155][156] Anticholinergic bronchodilators can also be used if a person cannot tolerate a SABA.[89] If a child requires admission to hospital additional ipratropium does not appear to help over a SABA.[157] For children over 2 years old with acute asthma symptoms, inhaled anticholinergic medications taken alone is safe but is not as effective as inhaled SABA or SABA combined with inhaled anticholinergic medication.[158][155] Adults who receive combined inhaled medications that includes short-acting anticholinergics and SABA may be at risk for increased adverse effects such as experiencing a tremor, agitation, and heart beat palpitations compared to people who are treated with SABA by itself.[156]
- Older, less selective adrenergic agonists, such as inhaled epinephrine, have similar efficacy to SABAs.[159] They are; however, not routinely recommended due to concerns regarding excessive cardiac stimulation.[160]
- A short course of corticosteroids after an acute asthma exacerbation may help prevent relapses and reduce hospitalizations.[161] For adults and children who are in the hospital due to acute asthma, systematic (IV) corticosteroids improve symptoms.[162][163]
Long–term control

- Corticosteroids are generally considered the most effective treatment available for long-term control.[131] Inhaled forms such as beclomethasone are usually used except in the case of severe persistent disease, in which oral corticosteroids may be needed.[131][164] For mild to moderate disease inhaled steroids may be used as needed.[165] For more severe disease it is recommended that inhaled formulations be used once or twice daily.[166]
- Long-acting beta-adrenoceptor agonists (LABA) such as salmeterol and formoterol can improve asthma control, at least in adults, when given in combination with inhaled corticosteroids.[167][168] In children this benefit is uncertain.[167][169][168] When used without steroids they increase the risk of severe side-effects,[170] and with corticosteroids they may slightly increase the risk.[171][172] Evidence suggests that for children who have persistent asthma, a treatment regime that includes LABA added to inhaled corticosteroids may improve lung function but does not reduce the amount of serious exacerbations.[173] Children who require LABA as part of their asthma treatment may need to go to the hospital more frequently.[173]
- Leukotriene receptor antagonists (anti-leukotriene agents such as montelukast and zafirlukast) may be used in addition to inhaled corticosteroids, typically also in conjunction with a LABA.[174][131][175][176][177] Evidence is insufficient to support use in acute exacerbations.[178][179] For adults or adolescents who have persistent asthma that is not controlled very well, the addition of anti-leukotriene agents along with daily inhaled corticosteriods improves lung function and reduces the risk of moderate and severe asthma exacerbations.[176] Anti-leukotriene agents may be effective alone for adolescents and adults, however there is no clear research suggesting which people with asthma would benefit from anti-leukotriene receptor alone.[180] In those under five years of age, anti-leukotriene agents were the preferred add-on therapy after inhaled corticosteroids by the British Thoracic Society in 2009.[181] A 2013 Cochrane systematic review concluded that anti-leukotriene agents appear to be of little benefit when added to inhaled steroids for treating children.[182] A similar class of drugs, 5-LOX inhibitors, may be used as an alternative in the chronic treatment of mild to moderate asthma among older children and adults.[174][183] As of 2013 there is one medication in this family known as zileuton.[174]
- Intravenous administration of the drug aminophylline does not provide an improvement in bronchodilation when compared to standard inhaled beta-2 agonist treatment.[184] Aminophylline treatment is associated with more adverse effects compared to inhaled beta-2 agonist treatment.[184]
- Mast cell stabilizers (such as cromolyn sodium) are another non-preferred alternative to corticosteroids.[131]
- For children with asthma which is well-controlled on combination therapy of inhaled corticosteroids (ICS) and long-acting beta2-agonists (LABA), the benefits and harms of stopping LABA and stepping down to ICS-only therapy are uncertain.[185] In adults who have stable asthma while they are taking a combination of LABA and inhaled corticosteroids (ICS), stopping LABA may increase the risk of asthma exacerbations that require treatment with corticosteroids by mouth.[186] Stopping LABA probably makes little or no important difference to asthma control or asthma-related quality of life.[186] Whether or not stopping LABA increases the risk of serious adverse events or exacerbations requiring an emergency department visit or hospitalisation is uncertain.[186]
- Anticholinergic medications such as ipratropium bromide have not been shown to be beneficial for treating chronic asthma in children over 2 years old,[187] but is not suggested for routine treatment of chronic asthma in adults.[188]
- There is no strong evidence to recommend chloroquine medication as a replacement for taking corticosteroids by mouth (for those who are not able to tolerate inhaled steroids).[189] Methotrexate is not suggested as a replacement for taking corticosteriods by mouth ("steroid sparing") due to the adverse effects associated with taking methotrexate and the minimal relief provided for asthma symptoms.[190]
Delivery methods
Medications are typically provided as metered-dose inhalers (MDIs) in combination with an asthma spacer or as a dry powder inhaler. The spacer is a plastic cylinder that mixes the medication with air, making it easier to receive a full dose of the drug. A nebulizer may also be used. Nebulizers and spacers are equally effective in those with mild to moderate symptoms. However, insufficient evidence is available to determine whether a difference exists in those with severe disease.[191] For delivering short-acting beta-agonists in acute asthma in children, spacers may have advantages compared to nebulisers, but children with life-threatening asthma have not been studied.[192] There is no strong evidence for the use of intravenous LABA for adults or children who have acute asthma.[193] There is insufficient evidence to directly compare the effectiveness of a metered-dose inhaler attached to a homemade spacer compared to commercially available spacer for treating children with asthma.[194]
Side effects
Long-term use of inhaled corticosteroids at conventional doses carries a minor risk of adverse effects.[195] Risks include thrush, the development of cataracts, and a slightly slowed rate of growth.[195][196][197] Rinsing the mouth after the use of inhaled steroids can decrease the risk of thrush.[198] Higher doses of inhaled steroids may result in lower bone mineral density.[199]
Others
Inflammation in the lungs can be estimated by the level of exhaled nitric oxide.[200][201] The use of exhaled nitric oxide levels (FeNO) to guide asthma medication dosing may have small benefits for preventing asthma attacks but the potential benefits are not strong enough for this approach to be universally recommended as a method to guide asthma therapy in adults or children.[200][201]
When asthma is unresponsive to usual medications, other options are available for both emergency management and prevention of flareups. Additional options include:
- Oxygen to alleviate hypoxia if saturations fall below 92%.[202]
- Corticosteroid by mouth are recommended with either five days of prednisone or 2 days of dexamethasone.[203][204] One review recommended a seven-day course of steroids.[205]
- Magnesium sulfate intravenous treatment increases bronchodilation when used in addition to other treatment in moderate severe acute asthma attacks.[8][206][207] In adults intravenous treatment results in a reduction of hospital admissions.[208] Low levels of evidence suggest that inhaled (nebulised) magnesium sulfate may have a small benefit for treating acute asthma in adults.[209] Overall, high quality evidence do not indicate a large benefit for combining magnesium sulfate with standard inhaled treatments for adults with asthma.[209]
- Heliox, a mixture of helium and oxygen, may also be considered in severe unresponsive cases.[8]
- Intravenous salbutamol is not supported by available evidence and is thus used only in extreme cases.[202]
- Methylxanthines (such as theophylline) were once widely used, but do not add significantly to the effects of inhaled beta-agonists.[202] Their use in acute exacerbations is controversial.[210]
- The dissociative anesthetic ketamine is theoretically useful if intubation and mechanical ventilation is needed in people who are approaching respiratory arrest; however, there is no evidence from clinical trials to support this.[211]
- For those with severe persistent asthma not controlled by inhaled corticosteroids and LABAs, bronchial thermoplasty may be an option.[212] It involves the delivery of controlled thermal energy to the airway wall during a series of bronchoscopies.[212][213] While it may increase exacerbation frequency in the first few months it appears to decrease the subsequent rate. Effects beyond one year are unknown.[214]
- Monoclonal antibody injections such as mepolizumab,[215] dupilumab,[216] or omalizumab may be useful in those with poorly controlled atopic asthma.[217] However, as of 2019 these medications are expensive and their use is therefore reserved for those with severe symptoms to achieve cost-effectiveness.[218] Monoclonal antibodies targeting interleukin-5 (IL-5) or its receptor (IL-5R), including mepolizumab, reslizumab or benralizumab, in addition to standard care in severe asthma is effective in reducing the rate of asthma exacerbations. There is limited evidence for improved health-related quality of life and lung function.[219]
- Evidence suggests that sublingual immunotherapy in those with both allergic rhinitis and asthma improve outcomes.[220]
- It is unclear if non-invasive positive pressure ventilation in children is of use as it has not been sufficiently studied.[221]
Alternative medicine
Many people with asthma, like those with other chronic disorders, use alternative treatments; surveys show that roughly 50% use some form of unconventional therapy.[222][223] There is little data to support the effectiveness of most of these therapies.
Evidence is insufficient to support the usage of vitamin C or vitamin E for controlling asthma.[224][225] There is tentative support for use of vitamin C in exercise induced bronchospasm.[226] Fish oil dietary supplements (marine n-3 fatty acids)[227] and reducing dietary sodium[228] do not appear to help improve asthma control. In people with mild to moderate asthma, treatment with vitamin D supplementation may reduce the risk of asthma exacerbations, however, it is not clear if this is only helpful for people who have low vitamin D levels to begin with (low baseline vitamin D).[229] There is no strong evidence to suggest that vitamin D supplements improve day-to-day asthma symptoms or a person's lung function.[229] There is no strong evidence to suggest that adults with asthma should avoid foods that contain monosodium glutamate (MSG).[230] There have not been enough high-quality studies performed to determine if children with asthma should avoid eating food that contains MSG.[230]
Acupuncture is not recommended for the treatment as there is insufficient evidence to support its use.[231][232] Air ionisers show no evidence that they improve asthma symptoms or benefit lung function; this applied equally to positive and negative ion generators.[233] Manual therapies, including osteopathic, chiropractic, physiotherapeutic and respiratory therapeutic maneuvers, have insufficient evidence to support their use in treating asthma.[234] The Buteyko breathing technique for controlling hyperventilation may result in a reduction in medication use; however, the technique does not have any effect on lung function.[132] Thus an expert panel felt that evidence was insufficient to support its use.[231] There is no clear evidence that breathing exercises are effective for treating children with asthma.[235]
Prognosis
The prognosis for asthma is generally good, especially for children with mild disease.[236] Mortality has decreased over the last few decades due to better recognition and improvement in care.[237] In 2010 the death rate was 170 per million for males and 90 per million for females.[238] Rates vary between countries by 100 fold.[238]
Globally it causes moderate or severe disability in 19.4 million people as of 2004 (16 million of which are in low and middle income countries).[239] Of asthma diagnosed during childhood, half of cases will no longer carry the diagnosis after a decade.[59] Airway remodeling is observed, but it is unknown whether these represent harmful or beneficial changes.[240] Early treatment with corticosteroids seems to prevent or ameliorates a decline in lung function.[241] Asthma in children also has negative effects on quality of life of their parents.[242]
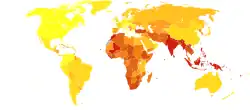 Asthma deaths per million persons in 20120–1011–1314–1718–2324–3233–4344–5051–6667–9596–251
Asthma deaths per million persons in 20120–1011–1314–1718–2324–3233–4344–5051–6667–9596–251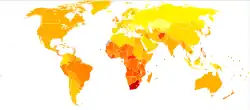 Disability-adjusted life year for asthma per 100,000 inhabitants in 2004.[243]no data0-100100–150150–200200–250250–300300–350350–400400–450450–500500–550550–600>600
Disability-adjusted life year for asthma per 100,000 inhabitants in 2004.[243]no data0-100100–150150–200200–250250–300300–350350–400400–450450–500500–550550–600>600
Epidemiology

In 2019, 262 million people globally had asthma, up from 183 million in 1990.[3][9] It caused about 455,000 deaths in 2019, most of which occurred in the developing world.[3] Asthma typically begins in childhood, more frequently before age 25 years, but the onset can be at any age.[1] Boys are affected more frequently than girls and women more than men.[1] Rates doubled over the second half of the 20th century, with higher rates in industrialized countries.[1] There appears to be lower rates in people growing up in farming environments.[1]
Global rates of asthma have increased significantly between the 1960s and 2008[244][245] with it being recognized as a major public health problem since the 1970s.[11] Rates of asthma have plateaued in the developed world since the mid-1990s with recent increases primarily in the developing world.[246] Asthma affects approximately 7% of the population of the United States[170] and 5% of people in the United Kingdom.[247] Canada, Australia and New Zealand have rates of about 14–15%.[248]
The average death rate from 2011 to 2015 from asthma in the UK was about 50% higher than the average for the European Union and had increased by about 5% in that time.[249] Children are more likely see a physician due to asthma symptoms after school starts in September.[250]
Economics
From 2000 to 2010, the average cost per asthma-related hospital stay in the United States for children remained relatively stable at about $3,600, whereas the average cost per asthma-related hospital stay for adults increased from $5,200 to $6,600.[251] In 2010, Medicaid was the most frequent primary payer among children and adults aged 18–44 years in the United States; private insurance was the second most frequent payer.[251] Among both children and adults in the lowest income communities in the United States there is a higher rate of hospital stays for asthma in 2010 than those in the highest income communities.[251]
History


Asthma was recognized in ancient Egypt and was treated by drinking an incense mixture known as kyphi.[10] It was officially named as a specific respiratory problem by Hippocrates circa 450 BC, with the Greek word for "panting" forming the basis of our modern name.[11] In 200 BC it was believed to be at least partly related to the emotions.[19] In the 12th century the Jewish physician-philosopher Maimonides wrote a treatise on asthma in Arabic, based partly on Arabic sources, in which he discussed the symptoms, proposed various dietary and other means of treatment, and emphasized the importance of climate and clean air.[252]
In 1873, one of the first papers in modern medicine on the subject tried to explain the pathophysiology of the disease while one in 1872, concluded that asthma can be cured by rubbing the chest with chloroform liniment.[253][254] Medical treatment in 1880 included the use of intravenous doses of a drug called pilocarpine.[255] In 1886, F. H. Bosworth theorized a connection between asthma and hay fever.[256] Epinephrine was first referred to in the treatment of asthma in 1905.[257] Oral corticosteroids began to be used for this condition in the 1950s while inhaled corticosteroids and selective short acting beta agonist came into wide use in the 1960s.[258][259]
A notable and well-documented case in the 19th century was that of young Theodore Roosevelt (1858–1919). At that time there was no effective treatment. Roosevelt's youth was in large part shaped by his poor health partly related to his asthma. He experienced recurring nighttime asthma attacks that caused the experience of being smothered to death, terrifying the boy and his parents.[260]
During the 1930s to 1950s, asthma was known as one of the "holy seven" psychosomatic illnesses. Its cause was considered to be psychological, with treatment often based on psychoanalysis and other talking cures.[261] As these psychoanalysts interpreted the asthmatic wheeze as the suppressed cry of the child for its mother, they considered the treatment of depression to be especially important for individuals with asthma.[261]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Drazen, Geoffrey M.; Bel, Elisabeth H. (2020). "81. Asthma". In Goldman, Lee; Schafer, Andrew I. (eds.). Goldman-Cecil Medicine. Vol. 1 (26th ed.). Philadelphia: Elsevier. pp. 527–535. ISBN 978-0-323-55087-1. Archived from the original on 2022-10-07. Retrieved 2022-10-07.
- 1 2 3 4 5 6 7 8 Mitchell, Ian; Govias, Gaynor (2021). "4. Clinical presentation of asthma". Asthma Education: Principles and Practice for the Asthma Educator (Second ed.). Switzerland: Springer. pp. 95–130. ISBN 978-3-030-77895-8. Archived from the original on 2022-10-07. Retrieved 2022-10-07.
- 1 2 3 4 5 6 7 8 9 10 11 "Asthma fact sheet". www.who.int. World Health Organization. 11 May 2022. Archived from the original on 5 March 2020. Retrieved 7 October 2022.
- 1 2 3 "Medications May Trigger Asthma Symptoms". www.aaaai.org. American Academy of Allergy Asthma and Immunology. 28 September 2020. Archived from the original on 9 July 2022. Retrieved 7 October 2022.
- ↑ CDC (27 September 2022). "Have Asthma? Learn how you can improve your health and quality of life". Centers for Disease Control and Prevention. Archived from the original on 6 October 2022. Retrieved 7 October 2022.
- ↑ "Asthma Fact sheet №307". WHO. November 2013. Archived from the original on June 29, 2011. Retrieved 3 March 2016.
- 1 2 3 4 NHLBI Guideline 2007, p. 214
- 1 2 3 NHLBI Guideline 2007, pp. 373–75
- 1 2 GBD 2015 Disease Injury Incidence Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- 1 2 Manniche L. (1999). Sacred luxuries: fragrance, aromatherapy, and cosmetics in ancient Egypt. Cornell University Press. pp. 49. ISBN 978-0-8014-3720-5.
- 1 2 3 4 5 6 7 8 9 10 11 12 Murray, John F. (2010). "Ch. 38 Asthma". In Mason, Robert J.; Murray, John F.; Broaddus, V. Courtney; Nadel, Jay A.; Martin, Thomas R.; King, Jr., Talmadge E.; Schraufnagel, Dean E. (eds.). Murray and Nadel's textbook of respiratory medicine (5th ed.). Elsevier. ISBN 978-1-4160-4710-0.
- ↑ Holgate, ST (July 2010). "A brief history of asthma and its mechanisms to modern concepts of disease pathogenesis". Allergy, asthma & immunology research. 2 (3): 165–71. doi:10.4168/aair.2010.2.3.165. ISSN 2092-7355. PMID 20592914. Archived from the original on 2022-08-01. Retrieved 2022-10-07.
- ↑ Jindal SK, ed. (2011). Textbook of pulmonary and critical care medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 242. ISBN 978-93-5025-073-0. Archived from the original on 2016-04-24.
- ↑ George, Ronald B. (2005). Chest medicine : essentials of pulmonary and critical care medicine (5th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 62. ISBN 978-0-7817-5273-2. Archived from the original on 2016-05-05.
- 1 2 3 GINA 2011, pp. 2–5
- ↑ GINA 2011, pp. 8–9
- ↑ Boulet LP (April 2009). "Influence of comorbid conditions on asthma". The European Respiratory Journal. 33 (4): 897–906. doi:10.1183/09031936.00121308. PMID 19336592.
- ↑ Boulet LP, Boulay MÈ (June 2011). "Asthma-related comorbidities". Expert Review of Respiratory Medicine. 5 (3): 377–93. doi:10.1586/ers.11.34. PMID 21702660.
- 1 2 editors, Andrew Harver, Harry Kotses (2010). Asthma, health and society a public health perspective. New York: Springer. p. 315. ISBN 978-0-387-78285-0. Archived from the original on 2016-05-12.
- ↑ Thomas M, Bruton A, Moffat M, Cleland J (September 2011). "Asthma and psychological dysfunction". Primary Care Respiratory Journal. 20 (3): 250–6. doi:10.4104/pcrj.2011.00058. PMC 6549858. PMID 21674122.
- ↑ Thomsen HS, Webb JA, eds. (2014). Contrast media : safety issues and ESUR guidelines (Third ed.). Dordrecht: Springer. p. 54. ISBN 978-3-642-36724-3. Archived from the original on 2020-07-26. Retrieved 2020-06-22.
- ↑ Agostini, BA; Collares, KF; Costa, FDS; Correa, MB; Demarco, FF (August 2019). "The role of asthma in caries occurrence - meta-analysis and meta-regression". The Journal of Asthma. 56 (8): 841–852. doi:10.1080/02770903.2018.1493602. PMID 29972654.
- 1 2 Thomas, MS; Parolia, A; Kundabala, M; Vikram, M (June 2010). "Asthma and oral health: a review". Australian Dental Journal. 55 (2): 128–33. doi:10.1111/j.1834-7819.2010.01226.x. PMID 20604752.
- ↑ Choudhry S, Seibold MA, Borrell LN, Tang H, Serebrisky D, Chapela R, et al. (July 2007). "Dissecting complex diseases in complex populations: asthma in latino americans". Proceedings of the American Thoracic Society. 4 (3): 226–33. doi:10.1513/pats.200701-029AW. PMC 2647623. PMID 17607004.
- ↑ Dietert RR (September 2011). "Maternal and childhood asthma: risk factors, interactions, and ramifications". Reproductive Toxicology. 32 (2): 198–204. doi:10.1016/j.reprotox.2011.04.007. PMID 21575714.
- ↑ Tan DJ, Walters EH, Perret JL, Lodge CJ, Lowe AJ, Matheson MC, Dharmage SC (February 2015). "Age-of-asthma onset as a determinant of different asthma phenotypes in adults: a systematic review and meta-analysis of the literature". Expert Review of Respiratory Medicine. 9 (1): 109–23. doi:10.1586/17476348.2015.1000311. PMID 25584929.
- ↑ Kelly FJ, Fussell JC (August 2011). "Air pollution and airway disease". Clinical and Experimental Allergy. 41 (8): 1059–71. doi:10.1111/j.1365-2222.2011.03776.x. PMID 21623970.
- ↑ GINA 2011, p. 6
- ↑ GINA 2011, p. 61
- 1 2 Gold DR, Wright R (2005). "Population disparities in asthma". Annual Review of Public Health. 26: 89–113. doi:10.1146/annurev.publhealth.26.021304.144528. PMID 15760282.
- ↑ American Lung Association (June 2001). "Urban air pollution and health inequities: a workshop report". Environmental Health Perspectives. 109 Suppl 3: 357–74. doi:10.1289/ehp.01109s3357. PMC 1240553. PMID 11427385.
- ↑ Brooks, Nancy; Sethi, Rajiv (February 1997). "The Distribution of Pollution: Community Characteristics and Exposure to Air Toxics". Journal of Environmental Economics and Management. 32 (2): 233–50. doi:10.1006/jeem.1996.0967.
- ↑ McGwin G, Lienert J, Kennedy JI (March 2010). "Formaldehyde exposure and asthma in children: a systematic review". Environmental Health Perspectives. 118 (3): 313–7. doi:10.1289/ehp.0901143. PMC 2854756. PMID 20064771.
- ↑ Jaakkola JJ, Knight TL (July 2008). "The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis". Environmental Health Perspectives. 116 (7): 845–53. doi:10.1289/ehp.10846. PMC 2453150. PMID 18629304.
- ↑ Bornehag CG, Nanberg E (April 2010). "Phthalate exposure and asthma in children". International Journal of Andrology. 33 (2): 333–45. doi:10.1111/j.1365-2605.2009.01023.x. PMID 20059582.
- ↑ Mamane A, Baldi I, Tessier JF, Raherison C, Bouvier G (June 2015). "Occupational exposure to pesticides and respiratory health". European Respiratory Review. 24 (136): 306–19. doi:10.1183/16000617.00006014. PMID 26028642.
- ↑ Mamane A, Raherison C, Tessier JF, Baldi I, Bouvier G (September 2015). "Environmental exposure to pesticides and respiratory health". European Respiratory Review. 24 (137): 462–73. doi:10.1183/16000617.00006114. PMID 26324808.
- ↑ Heintze K, Petersen KU (June 2013). "The case of drug causation of childhood asthma: antibiotics and paracetamol". European Journal of Clinical Pharmacology. 69 (6): 1197–209. doi:10.1007/s00228-012-1463-7. PMC 3651816. PMID 23292157.
- ↑ Henderson AJ, Shaheen SO (March 2013). "Acetaminophen and asthma". Paediatric Respiratory Reviews. 14 (1): 9–15, quiz 16. doi:10.1016/j.prrv.2012.04.004. PMID 23347656.
- ↑ Cheelo M, Lodge CJ, Dharmage SC, Simpson JA, Matheson M, Heinrich J, Lowe AJ (January 2015). "Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis". Archives of Disease in Childhood. 100 (1): 81–9. doi:10.1136/archdischild-2012-303043. PMID 25429049.
- ↑ Eyers S, Weatherall M, Jefferies S, Beasley R (April 2011). "Paracetamol in pregnancy and the risk of wheezing in offspring: a systematic review and meta-analysis". Clinical and Experimental Allergy. 41 (4): 482–9. doi:10.1111/j.1365-2222.2010.03691.x. PMID 21338428.
- ↑ van de Loo KF, van Gelder MM, Roukema J, Roeleveld N, Merkus PJ, Verhaak CM (January 2016). "Prenatal maternal psychological stress and childhood asthma and wheezing: a meta-analysis". The European Respiratory Journal. 47 (1): 133–46. doi:10.1183/13993003.00299-2015. PMID 26541526.
- ↑ Ahluwalia SK, Matsui EC (April 2011). "The indoor environment and its effects on childhood asthma". Current Opinion in Allergy and Clinical Immunology. 11 (2): 137–43. doi:10.1097/ACI.0b013e3283445921. PMID 21301330.
- ↑ Arshad SH (January 2010). "Does exposure to indoor allergens contribute to the development of asthma and allergy?". Current Allergy and Asthma Reports. 10 (1): 49–55. doi:10.1007/s11882-009-0082-6. PMID 20425514. S2CID 30418306.
- ↑ Custovic A, Simpson A (2012). "The role of inhalant allergens in allergic airways disease". Journal of Investigational Allergology & Clinical Immunology. 22 (6): 393–401, qiuz follow 401. PMID 23101182.
- 1 2 Gøtzsche PC, Johansen HK (April 2008). Gøtzsche PC (ed.). "House dust mite control measures for asthma". The Cochrane Database of Systematic Reviews (2): CD001187. doi:10.1002/14651858.CD001187.pub3. PMID 18425868.
- ↑ Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, Demoly P (July 2015). "Respiratory allergy caused by house dust mites: What do we really know?". The Journal of Allergy and Clinical Immunology. 136 (1): 38–48. doi:10.1016/j.jaci.2014.10.012. PMID 25457152.
- ↑ Sauni, Riitta; Verbeek, Jos H.; Uitti, Jukka; Jauhiainen, Merja; Kreiss, Kathleen; Sigsgaard, Torben (2015-02-25). "Remediating buildings damaged by dampness and mould for preventing or reducing respiratory tract symptoms, infections and asthma". The Cochrane Database of Systematic Reviews (2): CD007897. doi:10.1002/14651858.CD007897.pub3. ISSN 1469-493X. PMC 6769180. PMID 25715323.
- ↑ NHLBI Guideline 2007, pp. 11–12
- ↑ Ramsey CD, Celedón JC (January 2005). "The hygiene hypothesis and asthma". Current Opinion in Pulmonary Medicine. 11 (1): 14–20. doi:10.1097/01.mcp.0000145791.13714.ae. PMID 15591883.
- ↑ Bufford JD, Gern JE (May 2005). "The hygiene hypothesis revisited". Immunology and Allergy Clinics of North America. 25 (2): 247–62, v–vi. doi:10.1016/j.iac.2005.03.005. PMID 15878454.
- 1 2 Brooks C, Pearce N, Douwes J (February 2013). "The hygiene hypothesis in allergy and asthma: an update". Current Opinion in Allergy and Clinical Immunology. 13 (1): 70–7. doi:10.1097/ACI.0b013e32835ad0d2. PMID 23103806.
- ↑ Rao D, Phipatanakul W (October 2011). "Impact of environmental controls on childhood asthma". Current Allergy and Asthma Reports. 11 (5): 414–20. doi:10.1007/s11882-011-0206-7. PMC 3166452. PMID 21710109.
- ↑ Murk W, Risnes KR, Bracken MB (June 2011). "Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review". Pediatrics. 127 (6): 1125–38. doi:10.1542/peds.2010-2092. PMID 21606151.
- ↑ British Guideline 2009, p. 72
- ↑ Neu J, Rushing J (June 2011). "Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis". Clinics in Perinatology. 38 (2): 321–31. doi:10.1016/j.clp.2011.03.008. PMC 3110651. PMID 21645799.
- ↑ Von Hertzen LC, Haahtela T (February 2004). "Asthma and atopy - the price of affluence?". Allergy. 59 (2): 124–37. doi:10.1046/j.1398-9995.2003.00433.x. PMID 14763924.
- 1 2 Martinez FD (January 2007). "Genes, environments, development and asthma: a reappraisal". The European Respiratory Journal. 29 (1): 179–84. doi:10.1183/09031936.00087906. PMID 17197483.
- 1 2 3 Elward, Graham Douglas, Kurtis S. (2010). Asthma. London: Manson Pub. pp. 27–29. ISBN 978-1-84076-513-7. Archived from the original on 2016-05-17.
- 1 2 3 Ober C, Hoffjan S (March 2006). "Asthma genetics 2006: the long and winding road to gene discovery". Genes and Immunity. 7 (2): 95–100. doi:10.1038/sj.gene.6364284. PMID 16395390.
- ↑ Halapi E, Bjornsdottir US (January 2009). "Overview on the current status of asthma genetics". The Clinical Respiratory Journal. 3 (1): 2–7. doi:10.1111/j.1752-699X.2008.00119.x. PMID 20298365.
- ↑ Martinez FD (July 2007). "CD14, endotoxin, and asthma risk: actions and interactions". Proceedings of the American Thoracic Society. 4 (3): 221–5. doi:10.1513/pats.200702-035AW. PMC 2647622. PMID 17607003.
- 1 2 Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 978-1-4160-2999-1.
- ↑ GINA 2011, p. 4
- ↑ Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. (January 2013). "2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides". Arthritis and Rheumatism. 65 (1): 1–11. doi:10.1002/art.37715. PMID 23045170.
- ↑ Beuther DA (January 2010). "Recent insight into obesity and asthma". Current Opinion in Pulmonary Medicine. 16 (1): 64–70. doi:10.1097/MCP.0b013e3283338fa7. PMID 19844182.
- ↑ Holguin F, Fitzpatrick A (March 2010). "Obesity, asthma, and oxidative stress". Journal of Applied Physiology. 108 (3): 754–9. doi:10.1152/japplphysiol.00702.2009. PMID 19926826.
- ↑ Wood LG, Gibson PG (July 2009). "Dietary factors lead to innate immune activation in asthma". Pharmacology & Therapeutics. 123 (1): 37–53. doi:10.1016/j.pharmthera.2009.03.015. PMID 19375453.
- ↑ O'Rourke ST (October 2007). "Antianginal actions of beta-adrenoceptor antagonists". American Journal of Pharmaceutical Education. 71 (5): 95. doi:10.5688/aj710595. PMC 2064893. PMID 17998992.
- ↑ Salpeter S, Ormiston T, Salpeter E (2002). "Cardioselective beta-blockers for reversible airway disease". The Cochrane Database of Systematic Reviews (4): CD002992. doi:10.1002/14651858.CD002992. PMID 12519582.
- ↑ Morales DR, Jackson C, Lipworth BJ, Donnan PT, Guthrie B (April 2014). "Adverse respiratory effect of acute β-blocker exposure in asthma: a systematic review and meta-analysis of randomized controlled trials". Chest. 145 (4): 779–786. doi:10.1378/chest.13-1235. PMID 24202435.
- ↑ Lai T, Wu M, Liu J, Luo M, He L, Wang X, et al. (February 2018). "Acid-Suppressive Drug Use During Pregnancy and the Risk of Childhood Asthma: A Meta-analysis". Pediatrics. 141 (2): e20170889. doi:10.1542/peds.2017-0889. PMID 29326337.
- 1 2 3 4 Baxi SN, Phipatanakul W (April 2010). "The role of allergen exposure and avoidance in asthma". Adolescent Medicine. 21 (1): 57–71, viii–ix. PMC 2975603. PMID 20568555.
- ↑ Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ (January 2015). "Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors". The Journal of Allergy and Clinical Immunology. 135 (1): 110–22. doi:10.1016/j.jaci.2014.07.002. PMID 25159468.
- ↑ Chen E, Miller GE (November 2007). "Stress and inflammation in exacerbations of asthma". Brain, Behavior, and Immunity. 21 (8): 993–9. doi:10.1016/j.bbi.2007.03.009. PMC 2077080. PMID 17493786.
- ↑ Pike KC, Akhbari M, Kneale D, Harris KM (March 2018). Cochrane Airways Group (ed.). "Interventions for autumn exacerbations of asthma in children". The Cochrane Database of Systematic Reviews. 3: CD012393. doi:10.1002/14651858.CD012393.pub2. PMC 6494188. PMID 29518252.
- ↑ GINA 2011, p. 18
- ↑ Lemanske RF, Busse WW (February 2010). "Asthma: clinical expression and molecular mechanisms". The Journal of Allergy and Clinical Immunology. 125 (2 Suppl 2): S95-102. doi:10.1016/j.jaci.2009.10.047. PMC 2853245. PMID 20176271.
- 1 2 NHLBI Guideline 2007, p. 42
- ↑ GINA 2011, p. 20
- ↑ American Academy of Allergy, Asthma, and Immunology. "Five things physicians and patients should question" (PDF). Choosing Wisely. ABIM Foundation. Archived from the original (PDF) on November 3, 2012. Retrieved August 14, 2012.
- ↑ Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute (US). 2007. 07-4051. Archived from the original on 2021-11-19. Retrieved 2020-06-22 – via NCBI.
- ↑ Welsh EJ, Bara A, Barley E, Cates CJ (January 2010). Welsh EJ (ed.). "Caffeine for asthma" (PDF). The Cochrane Database of Systematic Reviews (1): CD001112. doi:10.1002/14651858.CD001112.pub2. PMC 7053252. PMID 20091514. Archived (PDF) from the original on 2019-11-30. Retrieved 2020-06-22.
- ↑ NHLBI Guideline 2007, p. 58
- ↑ Pinnock H, Shah R (April 2007). "Asthma". BMJ. 334 (7598): 847–50. doi:10.1136/bmj.39140.634896.BE. PMC 1853223. PMID 17446617.
- ↑ NHLBI Guideline 2007, p. 59
- 1 2 Yawn BP (September 2008). "Factors accounting for asthma variability: achieving optimal symptom control for individual patients" (PDF). Primary Care Respiratory Journal. 17 (3): 138–47. doi:10.3132/pcrj.2008.00004. PMC 6619889. PMID 18264646. Archived (PDF) from the original on 2009-03-26.
- 1 2 Moore WC, Pascual RM (June 2010). "Update in asthma 2009". American Journal of Respiratory and Critical Care Medicine. 181 (11): 1181–7. doi:10.1164/rccm.201003-0321UP. PMC 3269238. PMID 20516492.
- 1 2 Self, Timothy; Chrisman, Cary; Finch, Christopher (2009). "22. Asthma". In Mary Anne Koda-Kimble, Brian K. Alldredge; et al. (eds.). Applied therapeutics: the clinical use of drugs (9th ed.). Philadelphia: Lippincott Williams & Wilkins. OCLC 230848069.
- ↑ Delacourt C (June 2004). "[Bronchial changes in untreated asthma]" [Bronchial changes in untreated asthma]. Archives de Pediatrie. 11 Suppl 2 (Suppl. 2): 71s–73s. doi:10.1016/S0929-693X(04)90003-6. PMID 15301800.
- ↑ Schiffman, George (18 December 2009). "Chronic obstructive pulmonary disease". MedicineNet. Archived from the original on 28 August 2010. Retrieved 2 September 2010.
- 1 2 3 4 British Guideline 2009, p. 54
- ↑ Current Review of Asthma. London: Current Medicine Group. 2003. p. 42. ISBN 978-1-4613-1095-2. Archived from the original on 2017-09-08.
- ↑ Barnes, P. J. (2008). "Asthma". In Fauci, Anthony S.; Braunwald, E.; Kasper, D. L. (eds.). Harrison's Principles of Internal Medicine (17th ed.). New York: McGraw-Hill. pp. 1596–607. ISBN 978-0-07-146633-2.
- ↑ McMahon, Maureen (2011). Pediatrics a competency-based companion. Philadelphia, PA: Saunders/Elsevier. ISBN 978-1-4160-5350-7.
- ↑ Maitre B, Similowski T, Derenne JP (September 1995). "Physical examination of the adult patient with respiratory diseases: inspection and palpation". The European Respiratory Journal. 8 (9): 1584–93. PMID 8575588. Archived from the original on 2015-04-29.
- ↑ Werner HA (June 2001). "Status asthmaticus in children: a review". Chest. 119 (6): 1913–29. doi:10.1378/chest.119.6.1913. PMID 11399724.
- 1 2 Shiber JR, Santana J (May 2006). "Dyspnea". The Medical Clinics of North America. 90 (3): 453–79. doi:10.1016/j.mcna.2005.11.006. PMID 16473100.
- 1 2 Shah R, Saltoun CA (May–Jun 2012). "Chapter 14: Acute severe asthma (status asthmaticus)". Allergy and Asthma Proceedings. 33 Suppl 1 (3): 47–50. doi:10.2500/aap.2012.33.3547. PMID 22794687.
- 1 2 Khan DA (Jan–Feb 2012). "Exercise-induced bronchoconstriction: burden and prevalence". Allergy and Asthma Proceedings. 33 (1): 1–6. doi:10.2500/aap.2012.33.3507. PMID 22370526.
- ↑ Wuestenfeld JC, Wolfarth B (January 2013). "Special considerations for adolescent athletic and asthmatic patients". Open Access Journal of Sports Medicine. 4: 1–7. doi:10.2147/OAJSM.S23438. PMC 3871903. PMID 24379703.
- ↑ GINA 2011, p. 17
- ↑ Carlsen KH, Anderson SD, Bjermer L, Bonini S, Brusasco V, Canonica W, et al. (May 2008). European Respiratory, Society; European Academy of Allergy and Clinical, Immunology; GA(2)LEN. "Treatment of exercise-induced asthma, respiratory and allergic disorders in sports and the relationship to doping: Part II of the report from the Joint Task Force of European Respiratory Society (ERS) and European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA(2)LEN". Allergy. 63 (5): 492–505. doi:10.1111/j.1398-9995.2008.01663.x. PMID 18394123.
- ↑ Kindermann W (2007). "Do inhaled beta(2)-agonists have an ergogenic potential in non-asthmatic competitive athletes?". Sports Medicine. 37 (2): 95–102. doi:10.2165/00007256-200737020-00001. PMID 17241101. S2CID 20993439.
- ↑ Pluim BM, de Hon O, Staal JB, Limpens J, Kuipers H, Overbeek SE, et al. (January 2011). "β₂-Agonists and physical performance: a systematic review and meta-analysis of randomized controlled trials". Sports Medicine. 41 (1): 39–57. doi:10.2165/11537540-000000000-00000. PMID 21142283. S2CID 189906919.
- 1 2 3 Baur X, Aasen TB, Burge PS, Heederik D, Henneberger PK, Maestrelli P, et al. (June 2012). ERS Task Force on the Management of Work-related, Asthma. "The management of work-related asthma guidelines: a broader perspective". European Respiratory Review. 21 (124): 125–39. doi:10.1183/09059180.00004711. PMID 22654084.
- ↑ Kunnamo I, ed. (2005). Evidence-based medicine guidelines. Chichester: Wiley. p. 214. ISBN 978-0-470-01184-3.
- ↑ Frew AJ (2008). "Chapter 42: Occupational Asthma". In Castro M, Kraft M (eds.). Clinical Asthma. Philadelphia: Mosby / Elsevier. ISBN 978-0-323-07081-2.
- ↑ Chang JE, White A, Simon RA, Stevenson DD (2012). "Aspirin-exacerbated respiratory disease: burden of disease". Allergy and Asthma Proceedings. 33 (2): 117–21. doi:10.2500/aap.2012.33.3541. PMID 22525387.
- ↑ "Aspirin Exacerbated Respiratory Disease (AERD)". www.aaaai.org. American Academy of Allergy Asthma & Immunology. August 3, 2018. Archived from the original on September 18, 2018. Retrieved June 22, 2020.
- ↑ Kennedy JL, Stoner AN, Borish L (November 2016). "Aspirin-exacerbated respiratory disease: Prevalence, diagnosis, treatment, and considerations for the future". American Journal of Rhinology & Allergy. 30 (6): 407–413. doi:10.2500/ajra.2016.30.4370. PMC 5108840. PMID 28124651.
- 1 2 3 Adams KE, Rans TS (December 2013). "Adverse reactions to alcohol and alcoholic beverages". Annals of Allergy, Asthma & Immunology. 111 (6): 439–45. doi:10.1016/j.anai.2013.09.016. PMID 24267355.
- ↑ Peters SP (2014). "Asthma phenotypes: nonallergic (intrinsic) asthma". The Journal of Allergy and Clinical Immunology. In Practice. 2 (6): 650–2. doi:10.1016/j.jaip.2014.09.006. PMID 25439352.
- 1 2 NHLBI Guideline 2007, p. 46
- ↑ Lichtenstein, Richard (2013). Pediatric emergencies. Philadelphia: Elsevier. p. 1022. ISBN 978-0-323-22733-9. Archived from the original on 2017-09-08.
- ↑ Gibson PG, McDonald VM, Marks GB (September 2010). "Asthma in older adults". Lancet. 376 (9743): 803–13. doi:10.1016/S0140-6736(10)61087-2. PMID 20816547. S2CID 12275555.
- ↑ Hargreave FE, Parameswaran K (August 2006). "Asthma, COPD and bronchitis are just components of airway disease". The European Respiratory Journal. 28 (2): 264–7. doi:10.1183/09031936.06.00056106. PMID 16880365.
- ↑ Diaz, P. Knoell (2009). "23. Chronic obstructive pulmonary disease". Applied therapeutics: the clinical use of drugs (9th ed.). Philadelphia: Lippincott Williams & Wilkins.
- 1 2 NHLBI Guideline 2007, pp. 184–85
- ↑ "Asthma". World Health Organization. April 2017. Archived from the original on 29 June 2011. Retrieved 30 May 2017.
- ↑ Henneberger PK (April 2007). "Work-exacerbated asthma". Current Opinion in Allergy and Clinical Immunology. 7 (2): 146–51. doi:10.1097/ACI.0b013e328054c640. PMID 17351467.
- ↑ Lodge CJ, Allen KJ, Lowe AJ, Hill DJ, Hosking CS, Abramson MJ, Dharmage SC (2012). "Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: a systematic review of longitudinal studies". Clinical & Developmental Immunology. 2012: 176484. doi:10.1155/2012/176484. PMC 3251799. PMID 22235226.
- ↑ Chen CM, Tischer C, Schnappinger M, Heinrich J (January 2010). "The role of cats and dogs in asthma and allergy—a systematic review". International Journal of Hygiene and Environmental Health. 213 (1): 1–31. doi:10.1016/j.ijheh.2009.12.003. PMID 20053584.
- 1 2 Prescott SL, Tang ML (May 2005). Australasian Society of Clinical Immunology and, Allergy. "The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children". The Medical Journal of Australia. 182 (9): 464–7. doi:10.5694/j.1326-5377.2005.tb06787.x. PMID 15865590.
- ↑ Cates CJ, Rowe BH (February 2013). "Vaccines for preventing influenza in people with asthma". The Cochrane Database of Systematic Reviews. 2 (2): CD000364. doi:10.1002/14651858.CD000364.pub4. PMC 6999427. PMID 23450529.
- ↑ "Strategic Advisory Group of Experts on Immunization - report of the extraordinary meeting on the influenza A (H1N1) 2009 pandemic, 7 July 2009". Releve Epidemiologique Hebdomadaire. 84 (30): 301–4. July 2009. PMID 19630186.
- 1 2 Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A (May 2014). "Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis". Lancet. 383 (9928): 1549–60. doi:10.1016/S0140-6736(14)60082-9. PMID 24680633. S2CID 8532979.
- ↑ Ripoll, Brian C. Leutholtz, Ignacio (2011). Exercise and disease management (2nd ed.). Boca Raton: CRC Press. p. 100. ISBN 978-1-4398-2759-8. Archived from the original on 2016-05-06.
- ↑ Gibson, P. G.; Powell, H.; Coughlan, J.; Wilson, A. J.; Abramson, M.; Haywood, P.; Bauman, A.; Hensley, M. J.; Walters, E. H. (2003). "Self-management education and regular practitioner review for adults with asthma". The Cochrane Database of Systematic Reviews (1): CD001117. doi:10.1002/14651858.CD001117. ISSN 1469-493X. PMC 7032643. PMID 12535399.
- ↑ GINA 2011, p. 56
- 1 2 3 4 5 6 NHLBI Guideline 2007, p. 213
- 1 2 "British Guideline on the Management of Asthma" (PDF). Scottish Intercollegiate Guidelines Network. 2008. Archived (PDF) from the original on 19 August 2008. Retrieved 2008-08-04.
- 1 2 3 Kew KM, Nashed M, Dulay V, Yorke J (September 2016). "Cognitive behavioural therapy (CBT) for adults and adolescents with asthma". The Cochrane Database of Systematic Reviews. 9: CD011818. doi:10.1002/14651858.CD011818.pub2. PMC 6457695. PMID 27649894.
- ↑ Paudyal P, Hine P, Theadom A, Apfelbacher CJ, Jones CJ, Yorke J, et al. (May 2014). "Written emotional disclosure for asthma". The Cochrane Database of Systematic Reviews (5): CD007676. doi:10.1002/14651858.CD007676.pub2. PMID 24842151.
- ↑ Bhogal S, Zemek R, Ducharme FM (July 2006). "Written action plans for asthma in children". The Cochrane Database of Systematic Reviews (3): CD005306. doi:10.1002/14651858.CD005306.pub2. PMID 16856090.
- ↑ McCallum GB, Morris PS, Brown N, Chang AB (August 2017). "Culture-specific programs for children and adults from minority groups who have asthma". The Cochrane Database of Systematic Reviews. 8: CD006580. doi:10.1002/14651858.CD006580.pub5. PMC 6483708. PMID 28828760.
- ↑ Kew KM, Carr R, Donovan T, Gordon M (April 2017). "Asthma education for school staff". The Cochrane Database of Systematic Reviews. 4: CD012255. doi:10.1002/14651858.CD012255.pub2. PMC 6478185. PMID 28402017.
- ↑ Welsh EJ, Hasan M, Li P (October 2011). "Home-based educational interventions for children with asthma". The Cochrane Database of Systematic Reviews (10): CD008469. doi:10.1002/14651858.CD008469.pub2. PMID 21975783.
- ↑ Yorke J, Shuldham C (April 2005). "Family therapy for chronic asthma in children". The Cochrane Database of Systematic Reviews (2): CD000089. doi:10.1002/14651858.CD000089.pub2. PMC 7038646. PMID 15846599.
- ↑ Harris K, Kneale D, Lasserson TJ, McDonald VM, Grigg J, Thomas J (January 2019). Cochrane Airways Group (ed.). "School-based self-management interventions for asthma in children and adolescents: a mixed methods systematic review". The Cochrane Database of Systematic Reviews. 1: CD011651. doi:10.1002/14651858.CD011651.pub2. PMC 6353176. PMID 30687940.
- ↑ Kew KM, Malik P, Aniruddhan K, Normansell R (October 2017). "Shared decision-making for people with asthma". The Cochrane Database of Systematic Reviews. 10: CD012330. doi:10.1002/14651858.CD012330.pub2. PMC 6485676. PMID 28972652.
- ↑ Gatheral TL, Rushton A, Evans DJ, Mulvaney CA, Halcovitch NR, Whiteley G, et al. (April 2017). "Personalised asthma action plans for adults with asthma". The Cochrane Database of Systematic Reviews. 4: CD011859. doi:10.1002/14651858.CD011859.pub2. PMC 6478068. PMID 28394084.
- ↑ Welsh EJ, Carr R (September 2015). Cochrane Airways Group (ed.). "Pulse oximeters to self monitor oxygen saturation levels as part of a personalised asthma action plan for people with asthma". The Cochrane Database of Systematic Reviews (9): CD011584. doi:10.1002/14651858.CD011584.pub2. PMID 26410043.
- ↑ NHLBI Guideline 2007, p. 69
- ↑ Thomson NC, Spears M (February 2005). "The influence of smoking on the treatment response in patients with asthma". Current Opinion in Allergy and Clinical Immunology. 5 (1): 57–63. doi:10.1097/00130832-200502000-00011. PMID 15643345.
- ↑ Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH (2011). "Smoking and asthma". Journal of the American Board of Family Medicine. 24 (3): 313–22. doi:10.3122/jabfm.2011.03.100180. PMID 21551404.
- ↑ Singh M, Jaiswal N (June 2013). "Dehumidifiers for chronic asthma". The Cochrane Database of Systematic Reviews (6): CD003563. doi:10.1002/14651858.CD003563.pub2. PMID 23760885.
- ↑ Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ (September 2013). "Physical training for asthma". The Cochrane Database of Systematic Reviews. 9 (9): CD001116. doi:10.1002/14651858.CD001116.pub4. PMID 24085631.
- ↑ Yang ZY, Zhong HB, Mao C, Yuan JQ, Huang YF, Wu XY, et al. (April 2016). "Yoga for asthma". The Cochrane Database of Systematic Reviews. 4: CD010346. doi:10.1002/14651858.cd010346.pub2. PMC 6880926. PMID 27115477.
- ↑ Adeniyi FB, Young T (July 2012). "Weight loss interventions for chronic asthma". The Cochrane Database of Systematic Reviews (7): CD009339. doi:10.1002/14651858.CD009339.pub2. PMID 22786526.
- ↑ Cheng J, Pan T, Ye GH, Liu Q (July 2005). "Calorie controlled diet for chronic asthma". The Cochrane Database of Systematic Reviews (3): CD004674. doi:10.1002/14651858.CD004674.pub2. PMID 16034941.
- ↑ "QRG 153 • British guideline on the management of asthma" (PDF). SIGN. September 2016. Archived (PDF) from the original on 9 October 2016. Retrieved 6 October 2016.
- ↑ Normansell R, Sayer B, Waterson S, Dennett EJ, Del Forno M, Dunleavy A (June 2018). "Antibiotics for exacerbations of asthma". The Cochrane Database of Systematic Reviews. 6: CD002741. doi:10.1002/14651858.CD002741.pub2. PMC 6513273. PMID 29938789.
- ↑ Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. (May 2013). "An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction". American Journal of Respiratory and Critical Care Medicine. 187 (9): 1016–27. doi:10.1164/rccm.201303-0437ST. PMID 23634861.
- 1 2 Griffiths B, Ducharme FM (August 2013). "Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children". The Cochrane Database of Systematic Reviews (8): CD000060. doi:10.1002/14651858.CD000060.pub2. PMID 23966133.
- 1 2 Kirkland SW, Vandenberghe C, Voaklander B, Nikel T, Campbell S, Rowe BH (January 2017). "Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma". The Cochrane Database of Systematic Reviews. 1: CD001284. doi:10.1002/14651858.CD001284.pub2. PMC 6465060. PMID 28076656.
- ↑ Vézina K, Chauhan BF, Ducharme FM (July 2014). "Inhaled anticholinergics and short-acting beta(2)-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital". The Cochrane Database of Systematic Reviews. 7 (7): CD010283. doi:10.1002/14651858.CD010283.pub2. PMID 25080126.
- ↑ Teoh L, Cates CJ, Hurwitz M, Acworth JP, van Asperen P, Chang AB (April 2012). "Anticholinergic therapy for acute asthma in children" (PDF). The Cochrane Database of Systematic Reviews (4): CD003797. doi:10.1002/14651858.CD003797.pub2. PMID 22513916. Archived (PDF) from the original on 2021-08-27. Retrieved 2020-06-22.
- ↑ Rodrigo GJ, Nannini LJ (March 2006). "Comparison between nebulized adrenaline and beta2 agonists for the treatment of acute asthma. A meta-analysis of randomized trials". The American Journal of Emergency Medicine. 24 (2): 217–22. doi:10.1016/j.ajem.2005.10.008. PMID 16490653.
- ↑ NHLBI Guideline 2007, p. 351
- ↑ Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW (July 2007). "Corticosteroids for preventing relapse following acute exacerbations of asthma". The Cochrane Database of Systematic Reviews (3): CD000195. doi:10.1002/14651858.CD000195.pub2. PMID 17636617.
- ↑ Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH (2003). "Corticosteroids for hospitalised children with acute asthma". The Cochrane Database of Systematic Reviews (2): CD002886. doi:10.1002/14651858.CD002886. PMC 6999806. PMID 12804441.
- ↑ Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW (2001). "Early emergency department treatment of acute asthma with systemic corticosteroids". The Cochrane Database of Systematic Reviews (1): CD002178. doi:10.1002/14651858.CD002178. PMC 7025797. PMID 11279756.
- ↑ Adams N, Bestall J, Jones P (2001). "Beclomethasone at different doses for chronic asthma (review)". The Cochrane Database of Systematic Reviews (1): CD002879. doi:10.1002/14651858.CD002879. PMC 6999810. PMID 11279769.
- ↑ Cloutier, Michelle M.; Dixon, Anne E.; Krishnan, Jerry A.; Lemanske, Robert F.; Pace, Wilson; Schatz, Michael (3 December 2020). "Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update From the National Asthma Education and Prevention Program". JAMA. doi:10.1001/jama.2020.21974.
- ↑ NHLBI Guideline 2007, p. 218
- 1 2 Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ (May 2010). Ducharme FM (ed.). "Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children". The Cochrane Database of Systematic Reviews (5): CD005535. doi:10.1002/14651858.CD005535.pub2. PMC 4169792. PMID 20464739.
- 1 2 Ni Chroinin M, Greenstone I, Lasserson TJ, Ducharme FM (October 2009). "Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults and children". The Cochrane Database of Systematic Reviews (4): CD005307. doi:10.1002/14651858.CD005307.pub2. PMC 4170786. PMID 19821344.
- ↑ Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ (April 2010). Ducharme FM (ed.). "Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma". The Cochrane Database of Systematic Reviews (4): CD005533. doi:10.1002/14651858.CD005533.pub2. PMC 4169793. PMID 20393943.
- 1 2 Fanta CH (March 2009). "Asthma". The New England Journal of Medicine. 360 (10): 1002–14. doi:10.1056/NEJMra0804579. PMID 19264689.
- ↑ Cates CJ, Cates MJ (April 2012). Cates CJ (ed.). "Regular treatment with formoterol for chronic asthma: serious adverse events". The Cochrane Database of Systematic Reviews. 4 (4): CD006923. doi:10.1002/14651858.CD006923.pub3. PMC 4017186. PMID 22513944.
- ↑ Cates CJ, Cates MJ (July 2008). Cates CJ (ed.). "Regular treatment with salmeterol for chronic asthma: serious adverse events". The Cochrane Database of Systematic Reviews (3): CD006363. doi:10.1002/14651858.CD006363.pub2. PMC 4015854. PMID 18646149.
- 1 2 Chauhan BF, Chartrand C, Ni Chroinin M, Milan SJ, Ducharme FM (November 2015). "Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children". The Cochrane Database of Systematic Reviews (11): CD007949. doi:10.1002/14651858.CD007949.pub2. PMC 4167878. PMID 26594816.
- 1 2 3 Scott JP, Peters-Golden M (September 2013). "Antileukotriene agents for the treatment of lung disease". American Journal of Respiratory and Critical Care Medicine. 188 (5): 538–44. doi:10.1164/rccm.201301-0023PP. PMID 23822826.
- ↑ Chauhan BF, Ducharme FM (January 2014). "Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma" (PDF). The Cochrane Database of Systematic Reviews (1): CD003137. doi:10.1002/14651858.CD003137.pub5. PMID 24459050. Archived (PDF) from the original on 2020-09-30. Retrieved 2020-06-22.
- 1 2 Chauhan BF, Jeyaraman MM, Singh Mann A, Lys J, Abou-Setta AM, Zarychanski R, Ducharme FM (March 2017). "Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma". The Cochrane Database of Systematic Reviews. 3: CD010347. doi:10.1002/14651858.CD010347.pub2. PMC 6464690. PMID 28301050.
- ↑ Ducharme F, Schwartz Z, Hicks G, Kakuma R (2004). "Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma". The Cochrane Database of Systematic Reviews (2): CD003133. doi:10.1002/14651858.CD003133.pub2. PMID 15106191.
- ↑ GINA 2011, p. 74
- ↑ Watts K, Chavasse RJ (May 2012). Watts K (ed.). "Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children". The Cochrane Database of Systematic Reviews. 5 (5): CD006100. doi:10.1002/14651858.CD006100.pub2. PMID 22592708.
- ↑ Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM (November 2015). "Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis". Annals of Internal Medicine. 163 (10): 756–67. doi:10.7326/M15-1059. PMC 4648683. PMID 26390230.
- ↑ British Guideline 2009, p. 43
- ↑ Chauhan BF, Ben Salah R, Ducharme FM (October 2013). "Addition of anti-leukotriene agents to inhaled corticosteroids in children with persistent asthma". The Cochrane Database of Systematic Reviews (10): CD009585. doi:10.1002/14651858.CD009585.pub2. PMC 4235447. PMID 24089325.
- ↑ "Zyflo (Zileuton tablets)" (PDF). United States Food and Drug Administration. Cornerstone Therapeutics Inc. June 2012. p. 1. Archived (PDF) from the original on 13 December 2014. Retrieved 12 December 2014.
- 1 2 Nair P, Milan SJ, Rowe BH (December 2012). "Addition of intravenous aminophylline to inhaled beta(2)-agonists in adults with acute asthma". The Cochrane Database of Systematic Reviews. 12: CD002742. doi:10.1002/14651858.CD002742.pub2. PMC 7093892. PMID 23235591.
- ↑ Kew KM, Beggs S, Ahmad S (May 2015). "Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids". The Cochrane Database of Systematic Reviews (5): CD011316. doi:10.1002/14651858.CD011316.pub2. PMC 6486153. PMID 25997166. Archived from the original on 2020-07-26. Retrieved 2020-06-22.
- 1 2 3 Ahmad S, Kew KM, Normansell R (June 2015). "Stopping long-acting beta2-agonists (LABA) for adults with asthma well controlled by LABA and inhaled corticosteroids" (PDF). The Cochrane Database of Systematic Reviews (6): CD011306. doi:10.1002/14651858.CD011306.pub2. PMID 26089258. Archived (PDF) from the original on 2020-09-30. Retrieved 2020-06-22.
- ↑ McDonald NJ, Bara AI (2003). "Anticholinergic therapy for chronic asthma in children over two years of age". The Cochrane Database of Systematic Reviews (3): CD003535. doi:10.1002/14651858.CD003535. PMID 12917970.
- ↑ Westby M, Benson M, Gibson P (2004). "Anticholinergic agents for chronic asthma in adults". The Cochrane Database of Systematic Reviews (3): CD003269. doi:10.1002/14651858.CD003269.pub2. PMC 6483359. PMID 15266477.
- ↑ Dean T, Dewey A, Bara A, Lasserson TJ, Walters EH (2003). "Chloroquine as a steroid sparing agent for asthma". The Cochrane Database of Systematic Reviews (4): CD003275. doi:10.1002/14651858.CD003275. PMID 14583965.
- ↑ Davies H, Olson L, Gibson P (2000). "Methotrexate as a steroid sparing agent for asthma in adults". The Cochrane Database of Systematic Reviews (2): CD000391. doi:10.1002/14651858.CD000391. PMC 6483672. PMID 10796540.
- ↑ NHLBI Guideline 2007, p. 250
- ↑ Cates CJ, Welsh EJ, Rowe BH (September 2013). Cochrane Airways Group (ed.). "Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma". The Cochrane Database of Systematic Reviews (9): CD000052. doi:10.1002/14651858.CD000052.pub3. PMC 7032675. PMID 24037768.
- ↑ Travers AH, Milan SJ, Jones AP, Camargo CA, Rowe BH (December 2012). "Addition of intravenous beta(2)-agonists to inhaled beta(2)-agonists for acute asthma". The Cochrane Database of Systematic Reviews. 12: CD010179. doi:10.1002/14651858.CD010179. PMID 23235685.
- ↑ Rodriguez C, Sossa M, Lozano JM (April 2008). "Commercial versus home-made spacers in delivering bronchodilator therapy for acute therapy in children". The Cochrane Database of Systematic Reviews (2): CD005536. doi:10.1002/14651858.CD005536.pub2. PMC 6483735. PMID 18425921.
- 1 2 Rachelefsky G (January 2009). "Inhaled corticosteroids and asthma control in children: assessing impairment and risk". Pediatrics. 123 (1): 353–66. doi:10.1542/peds.2007-3273. PMID 19117903.
- ↑ Dahl R (August 2006). "Systemic side effects of inhaled corticosteroids in patients with asthma". Respiratory Medicine. 100 (8): 1307–17. doi:10.1016/j.rmed.2005.11.020. PMID 16412623.
- ↑ Thomas MS, Parolia A, Kundabala M, Vikram M (June 2010). "Asthma and oral health: a review". Australian Dental Journal. 55 (2): 128–33. doi:10.1111/j.1834-7819.2010.01226.x. PMID 20604752.
- ↑ Domino, Frank J.; Baldor, Robert A.; Golding, Jeremy; Grimes, Jill A. (2014). The 5-Minute Clinical Consult Premium 2015. Lippincott Williams & Wilkins. p. 192. ISBN 978-1-4511-9215-5. Archived from the original on 2021-08-27. Retrieved 2020-06-22.
- ↑ Skoner DP (December 2016). "Inhaled corticosteroids: Effects on growth and bone health". Annals of Allergy, Asthma & Immunology. 117 (6): 595–600. doi:10.1016/j.anai.2016.07.043. PMID 27979015.
- 1 2 Petsky HL, Kew KM, Turner C, Chang AB (September 2016). "Exhaled nitric oxide levels to guide treatment for adults with asthma". The Cochrane Database of Systematic Reviews. 9: CD011440. doi:10.1002/14651858.CD011440.pub2. PMC 6457753. PMID 27580628.
- 1 2 Petsky HL, Kew KM, Chang AB (November 2016). "Exhaled nitric oxide levels to guide treatment for children with asthma". The Cochrane Database of Systematic Reviews. 11: CD011439. doi:10.1002/14651858.CD011439.pub2. PMC 6432844. PMID 27825189.
- 1 2 3 Rodrigo GJ, Rodrigo C, Hall JB (March 2004). "Acute asthma in adults: a review". Chest. 125 (3): 1081–102. doi:10.1378/chest.125.3.1081. PMID 15006973.
- ↑ Moe, Samantha (9 May 2016). "#162 Dexamethasone Versus Prednisone for Pediatric Asthma: Will the results take your breath away?". CFPCLearn. Archived from the original on 25 March 2023. Retrieved 16 June 2023.
- ↑ Keeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, et al. (March 2014). "Dexamethasone for acute asthma exacerbations in children: a meta-analysis". Pediatrics. 133 (3): 493–9. doi:10.1542/peds.2013-2273. PMC 3934336. PMID 24515516.
- ↑ Rowe BH, Kirkland SW, Vandermeer B, Campbell S, Newton A, Ducharme FM, Villa-Roel C (March 2017). "Prioritizing Systemic Corticosteroid Treatments to Mitigate Relapse in Adults With Acute Asthma: A Systematic Review and Network Meta-analysis". Academic Emergency Medicine. 24 (3): 371–381. doi:10.1111/acem.13107. PMID 27664401. S2CID 30182169.
- ↑ Noppen M (August 2002). "Magnesium treatment for asthma: where do we stand?". Chest. 122 (2): 396–8. doi:10.1378/chest.122.2.396. PMID 12171805.
- ↑ Griffiths B, Kew KM (April 2016). "Intravenous magnesium sulfate for treating children with acute asthma in the emergency department" (PDF). The Cochrane Database of Systematic Reviews. 4: CD011050. doi:10.1002/14651858.CD011050.pub2. PMC 6599814. PMID 27126744. Archived (PDF) from the original on 2018-07-19. Retrieved 2020-06-22.
- ↑ Kew KM, Kirtchuk L, Michell CI (May 2014). Kew KM (ed.). "Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department" (PDF). The Cochrane Database of Systematic Reviews. 5 (5): CD010909. doi:10.1002/14651858.CD010909.pub2. PMID 24865567. Archived (PDF) from the original on 2017-09-21. Retrieved 2020-06-22.
- 1 2 Knightly R, Milan SJ, Hughes R, Knopp-Sihota JA, Rowe BH, Normansell R, Powell C (November 2017). "Inhaled magnesium sulfate in the treatment of acute asthma". The Cochrane Database of Systematic Reviews. 11: CD003898. doi:10.1002/14651858.CD003898.pub6. PMC 6485984. PMID 29182799.
- ↑ GINA 2011, p. 37
- ↑ NHLBI Guideline 2007, p. 399
- 1 2 Castro M, Musani AI, Mayse ML, Shargill NS (April 2010). "Bronchial thermoplasty: a novel technique in the treatment of severe asthma". Therapeutic Advances in Respiratory Disease. 4 (2): 101–16. doi:10.1177/1753465810367505. PMID 20435668.
- ↑ Boulet LP, Laviolette M (May–Jun 2012). "Is there a role for bronchial thermoplasty in the treatment of asthma?". Canadian Respiratory Journal. 19 (3): 191–2. doi:10.1155/2012/853731. PMC 3418092. PMID 22679610.
- ↑ GINA 2011, p. 70
- ↑ "Pulmonary-Allergy Drugs Advisory Committee Meeting". FDA. July 25, 2018. Archived from the original on July 26, 2020. Retrieved May 9, 2019.
- ↑ Sastre J, Dávila I (June 2018). "Dupilumab: A New Paradigm for the Treatment of Allergic Diseases". Journal of Investigational Allergology & Clinical Immunology. 28 (3): 139–150. doi:10.18176/jiaci.0254. PMID 29939132.
- ↑ Israel E, Reddel HK (September 2017). "Severe and Difficult-to-Treat Asthma in Adults". The New England Journal of Medicine. 377 (10): 965–976. doi:10.1056/NEJMra1608969. PMID 28877019. S2CID 44767865.
- ↑ McQueen RB, Sheehan DN, Whittington MD, van Boven JF, Campbell JD (August 2018). "Cost-Effectiveness of Biological Asthma Treatments: A Systematic Review and Recommendations for Future Economic Evaluations". PharmacoEconomics. 36 (8): 957–971. doi:10.1007/s40273-018-0658-x. PMID 29736895. S2CID 13681118.
- ↑ Farne HA, Wilson A, Powell C, Bax L, Milan SJ (September 2017). Cochrane Airways Group (ed.). "Anti-IL5 therapies for asthma". The Cochrane Database of Systematic Reviews. 9: CD010834. doi:10.1002/14651858.CD010834.pub3. PMC 6483800. PMID 28933516.
- ↑ Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, et al. (March 2013). "Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review". JAMA. 309 (12): 1278–88. doi:10.1001/jama.2013.2049. PMID 23532243.
- ↑ Korang SK, Feinberg J, Wetterslev J, Jakobsen JC (September 2016). "Non-invasive positive pressure ventilation for acute asthma in children". The Cochrane Database of Systematic Reviews. 9: CD012067. doi:10.1002/14651858.CD012067.pub2. PMC 6457810. PMID 27687114.
- ↑ Blanc PD, Trupin L, Earnest G, Katz PP, Yelin EH, Eisner MD (November 2001). "Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis : data from a population-based survey". Chest. 120 (5): 1461–7. doi:10.1378/chest.120.5.1461. PMID 11713120.
- ↑ Shenfield G, Lim E, Allen H (June 2002). "Survey of the use of complementary medicines and therapies in children with asthma". Journal of Paediatrics and Child Health. 38 (3): 252–7. doi:10.1046/j.1440-1754.2002.00770.x. PMID 12047692.
- ↑ Milan SJ, Hart A, Wilkinson M (October 2013). "Vitamin C for asthma and exercise-induced bronchoconstriction". The Cochrane Database of Systematic Reviews (10): CD010391. doi:10.1002/14651858.CD010391.pub2. PMC 6513466. PMID 24154977.
- ↑ Wilkinson M, Hart A, Milan SJ, Sugumar K (June 2014). "Vitamins C and E for asthma and exercise-induced bronchoconstriction". The Cochrane Database of Systematic Reviews (6): CD010749. doi:10.1002/14651858.CD010749.pub2. PMC 6513032. PMID 24936673.
- ↑ Hemilä H (June 2013). "Vitamin C may alleviate exercise-induced bronchoconstriction: a meta-analysis". BMJ Open. 3 (6): e002416. doi:10.1136/bmjopen-2012-002416. PMC 3686214. PMID 23794586.

- ↑ Woods, R. K.; Thien, F. C.; Abramson, M. J. (2002). "Dietary marine fatty acids (fish oil) for asthma in adults and children". The Cochrane Database of Systematic Reviews (3): CD001283. doi:10.1002/14651858.CD001283. ISSN 1469-493X. PMC 6436486. PMID 12137622.
- ↑ Pogson Z, McKeever T (March 2011). "Dietary sodium manipulation and asthma". The Cochrane Database of Systematic Reviews (3): CD000436. doi:10.1002/14651858.CD000436.pub3. PMC 7032646. PMID 21412865.
- 1 2 Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. (September 2016). "Vitamin D for the management of asthma" (PDF). The Cochrane Database of Systematic Reviews. 9: CD011511. doi:10.1002/14651858.CD011511.pub2. PMC 6457769. PMID 27595415. Archived (PDF) from the original on 2021-01-08. Retrieved 2020-06-22.
- 1 2 Zhou Y, Yang M, Dong BR (June 2012). "Monosodium glutamate avoidance for chronic asthma in adults and children". The Cochrane Database of Systematic Reviews (6): CD004357. doi:10.1002/14651858.CD004357.pub4. PMID 22696342.
- 1 2 NHLBI Guideline 2007, p. 240
- ↑ McCarney RW, Brinkhaus B, Lasserson TJ, Linde K (2004). McCarney RW (ed.). "Acupuncture for chronic asthma". The Cochrane Database of Systematic Reviews (1): CD000008. doi:10.1002/14651858.CD000008.pub2. PMC 7061358. PMID 14973944.
- ↑ Blackhall K, Appleton S, Cates CJ (September 2012). Blackhall K (ed.). "Ionisers for chronic asthma". The Cochrane Database of Systematic Reviews. 9 (9): CD002986. doi:10.1002/14651858.CD002986.pub2. PMC 6483773. PMID 22972060.
- ↑ Hondras MA, Linde K, Jones AP (April 2005). Hondras MA (ed.). "Manual therapy for asthma". The Cochrane Database of Systematic Reviews (2): CD001002. doi:10.1002/14651858.CD001002.pub2. PMID 15846609.
- ↑ Macêdo TM, Freitas DA, Chaves GS, Holloway EA, Mendonça KM (April 2016). "Breathing exercises for children with asthma". The Cochrane Database of Systematic Reviews. 4: CD011017. doi:10.1002/14651858.CD011017.pub2. PMC 7104663. PMID 27070225.
- ↑ Sergel, Michelle J.; Cydulka, Rita K. (September 2009). "Ch. 75: Asthma". In Wolfson, Allan B.; Harwood-Nuss, Ann (eds.). Harwood-Nuss' Clinical Practice of Emergency Medicine (5th ed.). Lippincott Williams & Wilkins. pp. 432–. ISBN 978-0-7817-8943-1. Archived from the original on 2020-07-26. Retrieved 2020-06-22.
- ↑ NHLBI Guideline 2007, p. 1
- 1 2 "The Global Asthma Report 2014". Archived from the original on 27 April 2016. Retrieved 10 May 2016.
- ↑ Organization, World Health (2008). The global burden of disease : 2004 update ([Online-Ausg.] ed.). Geneva: World Health Organization. p. 35. ISBN 978-92-4-156371-0.
- ↑ Maddox L, Schwartz DA (2002). "The pathophysiology of asthma". Annual Review of Medicine. 53: 477–98. doi:10.1146/annurev.med.53.082901.103921. PMID 11818486.
- ↑ Beckett PA, Howarth PH (February 2003). "Pharmacotherapy and airway remodelling in asthma?". Thorax. 58 (2): 163–74. doi:10.1136/thorax.58.2.163. PMC 1746582. PMID 12554904.
- ↑ Silva N, Carona C, Crespo C, Canavarro MC (June 2015). "Quality of life in pediatric asthma patients and their parents: a meta-analysis on 20 years of research". Expert Review of Pharmacoeconomics & Outcomes Research. 15 (3): 499–519. doi:10.1586/14737167.2015.1008459. hdl:10316/45410. PMID 25651982.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 11 November 2009. Retrieved November 11, 2009.
- ↑ Anandan C, Nurmatov U, van Schayck OC, Sheikh A (February 2010). "Is the prevalence of asthma declining? Systematic review of epidemiological studies". Allergy. 65 (2): 152–67. doi:10.1111/j.1398-9995.2009.02244.x. PMID 19912154.
- ↑ Grant EN, Wagner R, Weiss KB (August 1999). "Observations on emerging patterns of asthma in our society". The Journal of Allergy and Clinical Immunology. 104 (2 Pt 2): S1-9. doi:10.1016/S0091-6749(99)70268-X. PMID 10452783.
- ↑ Bousquet J, Bousquet PJ, Godard P, Daures JP (July 2005). "The public health implications of asthma". Bulletin of the World Health Organization. 83 (7): 548–54. PMC 2626301. PMID 16175830.
- ↑ Anderson HR, Gupta R, Strachan DP, Limb ES (January 2007). "50 years of asthma: UK trends from 1955 to 2004". Thorax. 62 (1): 85–90. doi:10.1136/thx.2006.066407. PMC 2111282. PMID 17189533.
- ↑ Masoli, Matthew (2004). Global Burden of Asthma (PDF). p. 9. Archived from the original (PDF) on 2013-05-02.
- ↑ "Asthma-related death rate in UK among highest in Europe, charity analysis finds". Pharmaceutical Journal. 3 May 2018. Archived from the original on 26 July 2020. Retrieved 13 August 2018.
- ↑ "Asthma attacks triple when children return to school in September". NHS UK. 3 July 2019. Archived from the original on 26 July 2020. Retrieved 23 August 2019.
- 1 2 3 Barrett ML, Wier LM, Washington R (January 2014). "Trends in Pediatric and Adult Hospital Stays for Asthma, 2000–2010". HCUP Statistical Brief #169. Rockville, MD: Agency for Healthcare Research and Quality. Archived from the original on 2014-03-28.
- ↑ Rosner, Fred (2002). "The Life of Moses Maimonides, a Prominent Medieval Physician" (PDF). Einstein Quart J Biol Med. 19 (3): 125–28. Archived (PDF) from the original on 2009-03-05.
- ↑ Thorowgood JC (November 1873). "On Bronchial Asthma". British Medical Journal. 2 (673): 600. doi:10.1136/bmj.2.673.600. PMC 2294647. PMID 20747287.
- ↑ Gaskoin G (March 1872). "On the Treatment of Asthma". British Medical Journal. 1 (587): 339. doi:10.1136/bmj.1.587.339. PMC 2297349. PMID 20746575.
- ↑ Berkart JB (June 1880). "The Treatment of Asthma". British Medical Journal. 1 (1016): 917–8. doi:10.1136/bmj.1.1016.917. PMC 2240555. PMID 20749537.
Berkart JB (June 1880). "The Treatment of Asthma". British Medical Journal. 1 (1017): 960–2. doi:10.1136/bmj.1.1017.960. PMC 2240530. PMID 20749546. - ↑ Bosworth FH (1886). "Hay Fever, Asthma, and Allied Affections". Transactions of the ... Annual Meeting of the American Climatological Association. American Climatological Association. Annual Meeting. 2: 151–70. PMC 2526599. PMID 21407325.
- ↑ Doig RL (February 1905). "Epinephrin; Especially in Asthma". California State Journal of Medicine. 3 (2): 54–5. PMC 1650334. PMID 18733372.
- ↑ von Mutius E, Drazen JM (March 2012). "A patient with asthma seeks medical advice in 1828, 1928, and 2012". The New England Journal of Medicine. 366 (9): 827–34. doi:10.1056/NEJMra1102783. PMID 22375974. S2CID 5143546.
- ↑ Crompton G (December 2006). "A brief history of inhaled asthma therapy over the last fifty years". Primary Care Respiratory Journal. 15 (6): 326–31. doi:10.1016/j.pcrj.2006.09.002. PMC 6730840. PMID 17092772.
- ↑ David McCullough (1981). Mornings on Horseback: The Story of an Extraordinary Family, a Vanished Way of Life and the Unique Child Who Became Theodore Roosevelt. Simon and Schuster. pp. 93–108. ISBN 978-0-7432-1830-6. Archived from the original on 2015-04-07.
- 1 2 Opolski M, Wilson I (September 2005). "Asthma and depression: a pragmatic review of the literature and recommendations for future research". Clinical Practice and Epidemiology in Mental Health. 1: 18. doi:10.1186/1745-0179-1-18. PMC 1253523. PMID 16185365.
Notes
- National Asthma Education and Prevention Program (2007). "Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma" (PDF). National Heart Lung and Blood Institute. Archived from the original (PDF) on 2013-10-19. Retrieved 2005-08-31.
- "British Guideline on the Management of Asthma" (PDF). British Thoracic Society. 2012 [2008]. Archived (PDF) from the original on 2008-08-19. Retrieved 2020-06-22.
- "Global Strategy for Asthma Management and Prevention" (PDF). Global Initiative for Asthma. 2011. Archived from the original (PDF) on 2012-11-20.
External links
| Classification | |
|---|---|
| External resources |