COVID-19 testing

| Part of a series on the |
| COVID-19 pandemic |
|---|
 Scientifically accurate atomic model of the external structure of SARS-CoV-2. Each "ball" is an atom. |
|

COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2. The two main branches detect either the presence of the virus or of antibodies produced in response to infection.[1][2] Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests (serology immunoassays) instead show whether someone once had the disease.[3] They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection.[4] It is used to assess disease prevalence, which aids the estimation of the infection fatality rate.[5]
Individual jurisdictions have adopted varied testing protocols, including whom to test, how often to test, analysis protocols, sample collection and the uses of test results.[6][7][8] This variation has likely significantly impacted reported statistics, including case and test numbers, case fatality rates and case demographics.[9][10][11][12] Because SARS-CoV-2 transmission occurs days after exposure (and before onset of symptoms), there is an urgent need for frequent surveillance and rapid availability of results.[13]
Test analysis is often performed in automated, high-throughput, medical laboratories by medical laboratory scientists. Alternatively, point-of-care testing can be done in physician's offices and parking lots, workplaces, institutional settings or transit hubs.
Methods

Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection.[15] Other techniques include a CT scan, checking for elevated body temperature, checking for low blood oxygen level, and the deployment of detection dogs at airports.[16][17][18]
Detection of the virus
Detection of the virus is usually done either by looking for the virus's inner RNA, or pieces of protein on the outside of the virus. Tests that look for the viral antigens (parts of the virus) are called antigen tests.
There are multiple types of tests that look for the virus by detecting the presence of the virus's RNA. These are called nucleic acid or molecular tests, after molecular biology. As of 2021, the most common form of molecular test is the reverse transcription polymerase chain reaction (RT-PCR) test.[19] Other methods used in molecular tests include CRISPR, isothermal nucleic acid amplification, digital polymerase chain reaction, microarray analysis, and next-generation sequencing.[19]
Reverse transcription polymerase chain reaction (RT-PCR) test
Polymerase chain reaction (PCR) is a process that amplifies (replicates) a small, well-defined segment of DNA many hundreds of thousands of times, creating enough of it for analysis. Test samples are treated with certain chemicals[20][21] that allow DNA to be extracted. Reverse transcription converts RNA into DNA.
Reverse transcription polymerase chain reaction (RT-PCR) first uses reverse transcription to obtain DNA, followed by PCR to amplify that DNA, creating enough to be analyzed.[21] RT-PCR can thereby detect SARS-CoV-2, which contains only RNA. The RT-PCR process generally requires a few hours.[22] These tests are also referred to as molecular or genetic assays.[3]
Real-time PCR (qPCR)[23] provides advantages including automation, higher-throughput and more reliable instrumentation. It has become the preferred method.[24][25]
The combined technique has been described as real-time RT-PCR[26] or quantitative RT-PCR[27] and is sometimes abbreviated qRT-PCR,[28] rRT-PCR[29] or RT-qPCR,[30] although sometimes RT-PCR or PCR are used. The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines propose the term RT-qPCR,[23] but not all authors adhere to this.
Average sensitivity for rapid molecular tests depend on the brand. For ID NOW, the average sensitivity was 73.0% with an average specificity of 99.7%; for Xpert Xpress the average sensitivity was 100% with an average specificity of 97.2%.[31]
In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa.

A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Samples can be obtained by various methods, including a nasopharyngeal swab, sputum (coughed up material),[32] throat swabs,[33] deep airway material collected via suction catheter[33] or saliva.[34][35] Drosten et al. remarked that for 2003 SARS, "from a diagnostic point of view, it is important to note that nasal and throat swabs seem less suitable for diagnosis, since these materials contain considerably less viral RNA than sputum, and the virus may escape detection if only these materials are tested."[36]
Sensitivity of clinical samples by RT-PCR is 63% for nasal swab, 32% for pharyngeal swab, 48% for feces, 72–75% for sputum, and 93–95% for bronchoalveolar lavage.[37]
The likelihood of detecting the virus depends on collection method and how much time has passed since infection. According to Drosten tests performed with throat swabs are reliable only in the first week. Thereafter the virus may abandon the throat and multiply in the lungs. In the second week, sputum or deep airways collection is preferred.[33]
Collecting saliva may be as effective as nasal and throat swabs,[34] although this is not certain.[38][35] Sampling saliva may reduce the risk for health care professionals by eliminating close physical interaction.[39] It is also more comfortable for the patient.[40] Quarantined people can collect their own samples.[39] A saliva test's diagnostic value depends on sample site (deep throat, oral cavity, or salivary glands).[35] Some studies have found that saliva yielded greater sensitivity and consistency when compared with swab samples.[41][42][43]
On 15 August 2020, the US FDA granted an emergency use authorization for a saliva test developed at Yale University that gives results in hours.[44][45]
On 4 January 2021, the US FDA issued an alert about the risk of false results, particularly false negative results, with the Curative SARS-Cov-2 Assay real-time RT-PCR test.[46]
Viral burden measured in upper respiratory specimens declines after symptom onset.[47] Following recovery, many patients no longer have detectable viral RNA in upper respiratory specimens. Among those who do, RNA concentrations three days following recovery are generally below the range in which replication-competent virus has been reliably isolated.[48] No clear correlation has been described between length of illness and duration of post-recovery shedding of viral RNA in upper respiratory specimens.[49]
.jpg.webp) Demonstration of a nasopharyngeal swab for COVID-19 testing
Demonstration of a nasopharyngeal swab for COVID-19 testing.jpg.webp) Demonstration of a throat swab for COVID-19 testing
Demonstration of a throat swab for COVID-19 testing A PCR machine
A PCR machine- Video of a nasopharyngeal swab for COVID-19 testing
Other molecular tests
Isothermal nucleic acid amplification tests also amplify the virus's genome. They are faster than PCR because they don't involve repeated heating and cooling cycles. These tests typically detect DNA using fluorescent tags, which are read out with specialized machines.
CRISPR gene editing technology was modified to perform the detection: if the CRISPR enzyme attaches to the sequence, it colors a paper strip. The researchers expect the resulting test to be cheap and easy to use in point-of-care settings.[50][51] The test amplifies RNA directly, without the RNA-to-DNA conversion step of RT-PCR.[52]
Antigen tests
.jpg.webp)
.jpg.webp)

An antigen is the part of a pathogen that elicits an immune response. Antigen tests look for antigen proteins from the viral surface. In the case of a coronavirus, these are usually proteins from the surface spikes.[53] SARS-CoV-2 antigens can be detected before onset of COVID-19 symptoms (as soon as SARS-CoV-2 virus particles) with more rapid test results, but with less sensitivity than PCR tests for the virus.[54]
Typical antigen tests are COVID-19 rapid antigen tests, often known as Lateral Flow Tests (LFT).
Antigen tests may be one way to scale up testing to much greater levels.[53] Isothermal nucleic acid amplification tests can process only one sample at a time per machine. RT-PCR tests are accurate but require too much time, energy and trained personnel to run the tests.[53] "There will never be the ability on a [PCR] test to do 300 million tests a day or to test everybody before they go to work or to school," Deborah Birx, head of the White House Coronavirus Task Force, said on 17 April 2020. "But there might be with the antigen test."[55]
Samples may be collected via nasopharyngeal swab, a swab of the anterior nares, or from saliva (obtained by various methods including lollipop tests for children).[56] The sample is then exposed to paper strips containing artificial antibodies designed to bind to coronavirus antigens. Antigens bind to the strips and give a visual readout. The process takes less than 30 minutes, can deliver results at point of care, and does not require expensive equipment or extensive training.[53]
Swabs of respiratory viruses often lack enough antigen material to be detectable.[57] This is especially true for asymptomatic patients who have little if any nasal discharge. Viral proteins are not amplified in an antigen test.[53][58] A Cochrane review based on 64 studies investigating the efficacy of 16 different antigen tests determined that they correctly identified COVID-19 infection in an average of 72% of people with symptoms, compared to 58% of people without symptoms.[59] Tests were most accurate (78%) when used in the first week after symptoms first developed, likely because people have the most virus in their system in the first days after they are infected.[59] While some scientists doubt whether an antigen test can be useful against COVID-19,[58] others have argued that antigen tests are highly sensitive when viral load is high and people are contagious, making them suitable for public health screening.[60][61] Routine antigen tests can quickly identify when asymptomatic people are contagious, while follow-up PCR can be used if confirmatory diagnosis is needed.[62]
Antibody tests
The body responds to a viral infection by producing antibodies that help neutralize the virus.[63] Blood tests (also called serology tests or serology immunoassays[3]) can detect the presence of such antibodies.[64] Antibody tests can be used to assess what fraction of a population has once been infected, which can then be used to calculate the disease's mortality rate.[5] They can also be used to determine how much antibody is contained in a unit of convalescent plasma, for COVID-19 treatment, or to verify if a given vaccine generates an adequate immune response.[65]
SARS-CoV-2 antibodies' potency and protective period have not been established.[5][66] Therefore, a positive antibody test may not imply immunity to a future infection. Further, whether mild or asymptomatic infections produce sufficient antibodies for a test to detect has not been established.[67] Antibodies for some diseases persist in the bloodstream for many years, while others fade away.[53]
The most notable antibodies are IgM and IgG. IgM antibodies are generally detectable several days after initial infection, although levels over the course of infection and beyond are not well characterized.[68] IgG antibodies generally become detectable 10–14 days after infection and normally peak around 28 days after infection.[69][70] This pattern of antibody development seen with other infections, often does not apply to SARS-CoV-2, however, with IgM sometimes occurring after IgG, together with IgG or not occurring at all.[71] Generally, however, median IgM detection occurs 5 days after symptom onset, whereas IgG is detected a median 14 days after symptom onset.[72] IgG levels significantly decline after two or three months.[73]
Genetic tests verify infection earlier than antibody tests. Only 30% of those with a positive genetic test produced a positive antibody test on day 7 of their infection.[67]
Antibody Test Types
Rapid diagnostic test (RDT)
RDTs typically use a small, portable, positive/negative lateral flow assay that can be executed at point of care. RDTs may process blood samples, saliva samples, or nasal swab fluids. RDTs produce colored lines to indicate positive or negative results.[74]
Enzyme-linked immunosorbent assay (ELISA)
ELISAs can be qualitative or quantitative and generally require a lab. These tests usually use whole blood, plasma, or serum samples. A plate is coated with a viral protein, such as a SARS-CoV-2 spike protein. Samples are incubated with the protein, allowing any antibodies to bind to it. The antibody-protein complex can then be detected with another wash of antibodies that produce a color/fluorescent readout.[74]
Neutralization assay
Neutralization assays assess whether sample antibodies prevent viral infection in test cells.[63] These tests sample blood, plasma or serum. The test cultures cells that allow viral reproduction (e.g., Vero E6 cells). By varying antibody concentrations, researchers can visualize and quantify how many test antibodies block virus replication.[74]
Chemiluminescent immunoassay
Chemiluminescent immunoassays are quantitative lab tests. They sample blood, plasma, or serum. Samples are mixed with a known viral protein, buffer reagents and specific, enzyme-labeled antibodies. The result is luminescent. A chemiluminescent microparticle immunoassay uses magnetic, protein-coated microparticles. Antibodies react to the viral protein, forming a complex. Secondary enzyme-labeled antibodies are added and bind to these complexes. The resulting chemical reaction produces light. The radiance is used to calculate the number of antibodies. This test can identify multiple types of antibodies, including IgG, IgM, and IgA.[74]
Neutralizing vis-à-vis binding antibodies
Most if not all large scale COVID-19 antibody testing looks for binding antibodies only and does not measure the more important neutralizing antibodies (NAb).[75][76][77] A NAb is an antibody that neutralizes the infectivity of a virus particle by blocking its attachment to or entry into a susceptible cell; enveloped viruses, like e.g. SARS-CoV-2, are neutralized by the blocking of steps in the replicative cycle up to and including membrane fusion.[78][63] A non-neutralizing antibody either does not bind to the crucial structures on the virus surface or binds but leaves the virus particle infectious; the antibody may still contribute to the destruction of virus particles or infected cells by the immune system.[79][63] It may even enhance infectivity by interacting with receptors on macrophages.[80] Since most COVID-19 antibody tests return a positive result if they find only binding antibodies, these tests cannot indicate that the subject has generated protective NAbs that protect against re-infection.[76][77]
It is expected that binding antibodies imply the presence of NAbs[77] and for many viral diseases total antibody responses correlate somewhat with NAb responses[81] but this is not established for COVID-19. A study of 175 recovered patients in China who experienced mild symptoms reported that 10 individuals had no detectable NAbs at discharge, or thereafter. How these patients recovered without the help of NAbs and whether they were at risk of re-infection was not addressed.[76] An additional source of uncertainty is that even if NAbs are present, viruses such as HIV can evade NAb responses.[75]
Studies have indicated that NAbs to the original SARS virus (the predecessor to the current SARS-CoV-2) can remain active for two years[82] and are gone after six years.[83] Nevertheless, memory cells including Memory B cells and Memory T cells[84] can last much longer and may have the ability to reduce reinfection severity.[83]
.jpg.webp) A point of care test in Peru. A blood droplet is collected by a pipette.
A point of care test in Peru. A blood droplet is collected by a pipette..jpg.webp) Blood from pipette is then placed onto a COVID-19 rapid diagnostic test device.
Blood from pipette is then placed onto a COVID-19 rapid diagnostic test device.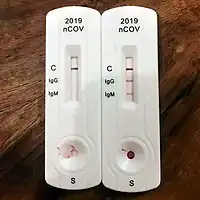
Other tests
Sniff tests
Sudden loss of smell can be used to screen people on a daily basis for COVID-19. A study by the National Institutes of Health showed that those infected with SARS-CoV-2 could not smell a 25% mixture of ethanol and water.[85] Because various conditions can lead to the loss of the sense of smell, a sniff test would not be definitive but indicate the need for a PCR test. Because the loss of the sense of smell shows up before other symptoms, there has been a call for widespread sniff testing.[86] Health care bureaucracies have generally ignored sniff tests even though they are quick, easy and capable of being self-administered daily. This has led some medical journals to write editorials supporting the adoption of sniff testing.[87]
Imaging
Typical visible features on CT initially include bilateral multilobar ground-glass opacities with a peripheral or posterior distribution.[88] COVID-19 can be identified with higher precision using CT than with RT-PCR.[89]
Subpleural dominance, crazy paving, and consolidation may develop as the disease evolves.[88][90] Chest CT scans and chest x-rays are not recommended for diagnosing COVID-19. Radiologic findings in COVID-19 lack specificity.[88][91]
Serology (CoLab score) tests
The standard blood test (quick scan) taken at the emergency room measures different values. By use of the blood quick scan the CoLab score is calculated with a developed algorithm based on how the coronavirus causes changes in the blood. The software is intended for use in emergency rooms to quickly rule out the presence of the disease in incoming patients. A not negative result is followed by a PCR (polymerase chain reaction) or LAMP (loop-mediated isothermal amplification) test.[92]
Breath tests
The breath test by a Coronavirus breathalyzer is a pre-screening test for people who have no or mild symptoms of COVID-19. A not negative result is followed by a PCR or LAMP test.
Animals
In May 2021, Reuters reported that Dutch researchers at Wageningen University had shown that trained bees could detect the virus in infected samples in seconds and this could benefit countries where test facilities are in short supply.[93] A two-month study by the Necker-Cochin hospital Paris in conjunction with the French national veterinary school also found that dogs were more reliable than current lateral flow tests according to the Guardian.[94]
Functional Assays
Tollotest is a molecular test that detects the activity of a COVID-19 protease, which is a biomarker for active infection.[95]
History

In January 2020, scientists from China published the first genetic sequences of SARS-CoV-2 via GISAID, a program that normally handled genetic sequence data.[97][98] Researchers around the world used that data to build molecular tests for the virus. Antigen- and antibody-based tests were developed later.
Even once the first tests were created, the supply was limited. As a result, no countries had reliable data on the prevalence of the virus early in the pandemic.[99] The WHO and other experts called for ramping up testing as the best way to slow the spread of the virus.[100][101] Shortages of reagent and other testing supplies became a bottleneck for mass testing in the EU, the UK and the US.[102][103][104] Early tests also encountered problems with reliability.[105][106]
Testing protocols
Drive-through testing
In drive-through testing, the person undergoing testing remains in a vehicle while a healthcare professional approaches the vehicle and obtains a sample, all while taking appropriate precautions such as wearing personal protective equipment (PPE).[107][108] Drive-through centers helped South Korea accelerate its testing program.[109]
Home collection

In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.[110]
Pooled testing
Pooled testing can improve turnaround time, by combining a number of samples to be tested together. If the pool result is negative, all samples are negative. If the test result is positive, samples will need to be individually tested.[65]
In Israel, researchers at Technion and Rambam Hospital developed a method for testing samples from 64 patients simultaneously, by pooling the samples and only testing further if the combined sample was positive.[111][112][113] Pool testing was then adopted in Israel, Germany, Ghana[114][115][116] South Korea,[117] Nebraska,[118] China[119] and the Indian states of Uttar Pradesh,[120] West Bengal,[121] Punjab,[122] Chhattisgarh[123] and Maharashtra.[124]
Open source, multiplexed designs released by Origami Assays can test as many as 1122 patient samples using only 93 assays.[125] These balanced designs can be run in small laboratories without robotic liquid handlers.
Multi-tiered testing
One study proposed a rapid immune response assay as a screening test, with a confirmatory nucleic acid test for diagnosis, followed by a rapid antibody test to determine course of action and assess population exposure/herd immunity.[126]
Required volume
Required testing levels are a function of disease spread. The more the cases, the more tests are needed to manage the outbreak. COVID-19 tends to grow exponentially at the beginning of an outbreak, meaning that the number of required tests initially also grows exponentially. If properly targeted testing grows more rapidly than cases, it can be contained.
WHO recommends increasing testing until fewer than 10% are positive in any given jurisdiction.[127]
United States

Blue: CDC lab
Orange: Public health lab
Gray: Data incomplete due to reporting lag
Not shown: Testing at private labs; total exceeded 100,000 per day by 27 March.[128]
Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy.[129] The Edmond J. Safra Center for Ethics estimated on 4 April that this capacity could be available by late July.[130] Romer pointed to single-molecule real-time sequencing equipment from Pacific Biosciences[129][131] and to the Ion Torrent Next-Generation Sequencing equipment from ThermoFisher Scientific.[129][132] According to Romer, "Recent research papers suggest that any one of these has the potential to scale up to millions of tests per day." This plan requires removing regulatory hurdles. Romer estimated that $100 billion would cover the costs.[129]
Romer also claimed that high test accuracy is not required if tests are administered frequently enough. He ran model simulations in which 7% of the population is tested every day using a test with a 20% false negative rate and a 1% false positive rate. The average person would be tested roughly every two weeks. Those who tested positive would go into quarantine. Romer's simulation indicated that the fraction of the population that is infected at any given time (known as the attack rate) peaks reaches roughly 8% in about thirty days before gradually declining, in most runs reaching zero at 500 days, with cumulative prevalence remaining below 20%.[133]
Snapshot mass-testing
A study found that, despite possibly suboptimal implementation, the snapshot mass-testing approach conducted by Slovakia by which ~80% of its population was tested for COVID-19 within a weekend at the end of October 2020 was highly efficacious, decreasing observed prevalence by 58% within one week and by 70% compared to a hypothetical scenario of no snapshot mass-testing.[134][135] The significant reduction resulted from a set of complementary lockdown and quarantine measures whereby citizens who tested positive were quarantined synchronously the weeks afterwards.[136]
Surveillance and screening of populations
As of August 2020, the WHO recognizes wastewater surveillance of SARS-CoV-2 as a potentially useful source of information on the prevalence and temporal trends of COVID-19 in communities, while highlighting that gaps in research such as viral shedding characteristics should be addressed.[137] Such aggregative testing may have detected early cases.[138] Studies show that wastewater-based epidemiology has the potential for an early warning system and monitoring for COVID-19 infections.[139][140][141][142][143] This may prove particularly useful once large shares of regional populations are vaccinated or recovered and don't need to conduct rapid tests while in some cases being infectious nevertheless.[144]
Available tests

Countries around the world developed tests independently and in partnership with others.
Nucleic acid tests
Tests developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the US targeted different parts of the viral genome. WHO adopted the German system for manufacturing kits sent to low-income countries without the resources to develop their own.
PowerChek Coronavirus looks for the "E" gene shared by all beta coronaviruses, and the RdRp gene specific to SARS-CoV-2.[145]
.jpg.webp)
.jpg.webp)
Abbott Laboratories' ID Now nucleic acid test uses isothermal amplification technology.[146] The assay amplifies a unique region of the virus's RdRp gene; the resulting copies are then detected with "fluorescently-labeled molecular beacons".[147] The test kit uses the company's "toaster-size" ID Now device, which is widely deployed in the US.[148] The device can be used in laboratories or in point of care settings, and provides results in 13 minutes or less.[147]
Primerdesign offers its Genesig Real-Time PCR Coronavirus (COVID‑19). Cobas SARS-CoV-2 Qualitative assay runs on the Cobas® 6800/8800 Systems by Roche Molecular Systems. They are offered by the United Nations and other procurement agencies.
Antigen tests

Quidel's "Sofia 2 SARS Antigen FIA"[62][149] is a lateral flow test that uses monoclonal antibodies to detect the virus's nucleocapsid (N) protein.[150] The result is read out by the company's Sofia 2 device using immunofluorescence.[150] The test is simpler and cheaper but less accurate than nucleic acid tests. It can be deployed in laboratories or at point of care and gives results in 15 minutes.[149] A false negative result occurs if the sample's antigen level is positive but below the test's detection limit, requiring confirmation with a nucleic acid test.[150]
The Innova SARS-CoV-2 Antigen Rapid Qualitative Test was never approved for use in the United States, but was being sold by the company anyway. The FDA inspected Innova facilities in California in March and April 2021, and found inadequate quality assurance of tests manufactured in China.[151] On 23 April 2021, the company issued a recall. The FDA warned consumers to return or destroy the devices because the rate of false positives and false negatives found in clinical trials were higher than the rate claimed by the packaging.[152] Over 1 billion tests from the company have been distributed in the UK, with £3 billion in funding as part of Operation Moonshot, and the MHRK has authorized exceptional use until at least 28 August 2021.[151] Concerned experts pointed out that accuracy dropped significantly when screening was conducted by the public instead of by a medical professional, and that the test was not designed to screen asymptomatic people.[151] A 2020 study found 79% of positive cases were found when used by laboratory scientists, but only 58% when used by the general public and 40% when used for city-wide screening in Liverpool.[153]
Serology (antibody) tests
Antibodies are usually detectable 14 days after the onset of the infection. Multiple jurisdictions survey their populations using these tests.[154][155] The test requires a blood sample.
Private US labs including Quest Diagnostics and LabCorp offer antibody testing upon request.[156]
Certain antibody tests are available in several European countries and also in the US.[157][158] Quotient Limited developed a CE marked antibody test that also received an US FDA Emergency Use Authorization.[159][160][161][162]
Roche offers a selective ELISA serology test.[163]
A summary review in BMJ has noted that while some "serological tests … might be cheaper and easier to implement at the point of care [than RT-PCR]", and such testing can identify previously infected individuals, "caution is warranted … using serological tests for … epidemiological surveillance". The review called for higher quality studies assessing accuracy with reference to a standard of "RT-PCR performed on at least two consecutive specimens, and, when feasible, includ[ing] viral cultures."[164][165] CEBM researchers have called for in-hospital 'case definition' to record "CT lung findings and associated blood tests"[166] and for the WHO to produce a "protocol to standardise the use and interpretation of PCR" with continuous re-calibration.[167]
Accuracy
| Samples source | Positive rate |
|---|---|
| Bronchoalveolar lavage fluid specimens | 93% (14/15) |
| Sputum | 72% (75/104) |
| Nasal swabs | 63% (5/8) |
| Fibrobronchoscope brush biopsy | 46% (6/13) |
| Pharyngeal swabs | 32% (126/398) |
| Feces | 29% (44/153) |
| Blood | 1% (3/307) |
Accuracy is measured in terms of specificity and selectivity. Test errors can be false positives (the test is positive, but the virus is not present) or false negatives, (the test is negative, but the virus is present).[169]
Sensitivity and specificity
Sensitivity indicates whether the test accurately identifies whether the virus is present. Each test requires a minimum level of viral load in order to produce a positive result. A 90% sensitive test will correctly identify 90% of infections, missing the other 10% (a false negative). Even relatively high sensitivity rates can produce high rates of false negatives in populations with low incidence rates.[169]
In a diagnostic test, sensitivity is a measure of how well a test can identify true positives and specificity is a measure of how well a test can identify true negatives. For all testing, both diagnostic and screening, there is usually a trade-off between sensitivity and specificity, such that higher sensitivities will mean lower specificities and vice versa.

A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.
Low-specificity tests have a low positive predictive value (PPV) when prevalence is low. For example, suppose incidence is 5%. Testing 100 people at random using a test that has a specificity of 95% would yield on average 5 people who are actually negative who would incorrectly test positive. Since 5% of the subjects actually are positive, another five would also test positive correctly, totaling 10 positive results. Thus, the PPV is 50%,[170] an outcome no different from a coin toss. In this situation retesting those with a positive result increases the PPV to 94.5%, meaning that only 4.5% of the second tests would return the incorrect result, on average less than 1 incorrect result.[171]
Causes of test error
The time course of infection affects the accuracy of some tests. Samples may be collected before the virus has had a chance to establish itself or after the body has begun to eliminate it. A May 2020 review of PCR-RT testing found that the median probability of a false-negative result decreased from 100% on day 1, to 67% on day 4. On the day of symptom onset, the probability was 38%, which decreased to 20% 3 days later.[172]
PCR-based test
.png.webp)
RT-PCR is the most commonly-used diagnostic test.[173] PCR tests by nasopharyngeal swab have a sensitivity of 73%, but systematic analysis of specificity has not been determined due to the lack of PCR studies with a control group.[174]
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six since symptom onset.[175][176]
Sensitivity is also a function of the number of PCR cycles, as well as time and temperature between sample collection and analysis.[177] A cycle threshold of 20 cycles would be adequate to detect SARS-Cov-2 in a highly infective person.[177] Cycle thresholds above 34 are increasingly likely to give false positives outside of high biosafety level facilities.[177]
On July 16, 2020, Dr. Anthony Fauci of the US CDC indicated that positive results obtained from RT-PCR tests run at more than 35 cycles were almost always "just dead nucleotides".[178] On August 29, 2020, the New York Times reported that, "In three sets of testing data that include cycle thresholds, compiled by officials in Massachusetts, New York and Nevada … most tests set the limit at 40 [cycles], a few at 37” and that the CDC was examining the use of cycle threshold measures “for policy decisions,”[179] On July 21, 2021, the CDC, in their "Real-Time RT-PCR Diagnostic Pan: Instructions for Use", indicated tests results should be determined at 40 cycles.[180]
A Dutch CDC-led laboratory investigation compared 7 PCR kits.[181] Test kits made by BGI, R-Biopharm AG, BGI, KH Medical and Seegene showed high sensitivity.[182]
High sensitivity kits are recommended to assess people without symptoms, while lower sensitivity tests are adequate when diagnosing symptomatic patients.[181]
The University of Oxford's Centre for Evidence-Based Medicine (CEBM) has pointed to mounting evidence[183][184] that "a good proportion of 'new' mild cases and people re-testing positives via RT-PCR after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with", and have called for "an international effort to standardize and periodically calibrate testing".[185] On 7 September, the UK government issued "guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results".[186]
On 4 January 2021, the US FDA issued an alert about the risk of false results, particularly false negative results, with the Curative SARS-Cov-2 Assay real-time RT-PCR test.[46]
Isothermal nucleic amplification test
One study reported that the ID Now COVID-19 test showed sensitivity of 85.2%. Abbott responded that the issue could have been caused by analysis delays.[187] Another study rejected the test in their clinical setting because of this low sensitivity.[188]
Confirmatory testing
The WHO recommends countries that do not have testing capacity and national laboratories with limited experience on COVID‑19 send their first five positives and the first ten negative COVID‑19 samples to one of the 16 WHO reference laboratories for confirmatory testing.[189][190] Out of the sixteen reference laboratories, seven are in Asia, five in Europe, two in Africa, one in North America and one in Australia.[191]
National responses
Iceland
Iceland managed the pandemic with aggressive contact tracing, inbound travel restrictions, testing, and quarantining, but with less aggressive lock-downs.[192]
India
Italy
Researchers tested the entire population of Vò, the site of Italy's first COVID‑19 death. They tested about 3,400 people twice, at an interval of ten days. About half the people testing positive had no symptoms. All discovered cases were quarantined. Along with restricting travel to the commune, new infections were eliminated.[193]
Japan
Unlike other Asian countries, Japan did not experience a pandemic of SARS or MERS, so the country's PCR testing system was not well developed.[194][195] Japan preferentially tested patients with severe illness and their close contacts at the beginning. Japan's Novel Coronavirus Expert Meeting chose cluster measures to identify infections clusters.[194][195] The Expert Meeting analyzed the outbreak from Wuhan and identified conditions leading to clusters (closed spaces, crowded spaces and close-contact), and asked people to avoid them.[195][196]
In January, contact tracers took action shortly after the first infection was found. Only administrative tests were carried out at first, until insurance began covering PCR tests on 6 March. Private companies began to test, and the test system gradually expanded.[194][197]
On 3 April, those with positive tests were legally permitted to recuperate at home or in a hotel if they had asymptomatic or mild illness, ending the hospital bed shortage.[198] The first wave (from China) was contained,[199] but a second wave (caused by returnees from Europe and the US) in mid-March led to spreading infection in April.[195] On 7 April, Japan declared a state of emergency, (less strict than a lockdown, because it did not block cities or restrict outings).[195][198][200] On 13 May, antigen test kits became covered by insurance, and were combined with a PCR test for diagnosis.[201][202]
Japan's PCR test count per capita remained far smaller than in some other countries even though its positive test rate was lower. Excess mortality was observed in March.[196][200][203] The Expert Meeting stated, "The Japanese health care system originally carries out pneumonia surveillance, allowing it to detect most of the severely ill patients who develop pneumonia. There are a large number of CT scanners in Japan and they have spread to small hospitals all over the country, so pneumonia patients are rarely missed. In that sense, it meets the same standards as other countries that mainly carry out PCR tests."[196][203] The group recommended using CT scans data and doctor's findings for diagnosis.[204][205] On the Diamond Princess cruise ship, many people who initially tested negative later tested positive. Half of coronavirus-positives there who remained mild or asymptomatic had pneumonia findings on CT scans and their CT image showed a frosted glass shadow that is characteristic of infection.[204][206]
As of 18 July, Japan's daily PCR testing capacity was about 32,000, more than three times the 10,000 cases as of April. When the antigen test is added to it, the number is about 58,000. The number of tests per 1,000 people in the United States is about 27 times that of Japan, the UK is 20 times, Italy is 8 times, and South Korea is twice (as of 26 July).[207][208][209] The number of those infected with coronavirus and inpatients has increased in July, but the number of serious cases has not increased. This is thought to be due to the proper testing of those infected in July compared to those in April. In April, the number of tests could not catch up with the increase in the number of infected people, and the test standards were strict, so the test positive rate exceeded 30% at the peak. It means that there were quite a few cases where the those infected was not PCR tested. It is thought that the severe case was preferentially tested though there were a lot of mild cases and asymptomatic carriers mainly in the young during the first wave. In other words, it became possible to grasp the actual situation of infection much better than before by strengthening the testing system.[210] At the end of July, accommodation facilities for mild and asymptomatic carriers became full, and the authorities requested hospitals to prepare beds for the mild. However, it became difficult to treat patients with other illnesses and to maintain the ICU system including the staff due to the occupation of hospital beds by patients with mild symptoms.[211][212][213]
Russia
On 27 April 2020, Russia tested 3 million people and had 183,000 positive results.[214] On 28 April Anna Popova, head of Federal Service for Surveillance in Healthcare (Roszdravnadzor) stated that 506 laboratories were testing; that 45% of those who tested positive had no symptoms; that 5% of patients had a severe form; and 40% of infections were from family members. Illness improved from six days to one day after symptoms appeared. Antibody testing was carried out on 3,200 Moscow doctors, finding 20% immunity.[215]
Singapore
With contact tracing, inbound travel restrictions, testing, and quarantining, Singapore arrested the initial spread without complete lockdown.[216]
Slovakia
In late October 2020 Slovakia tested 3.62 million people in a weekend, from a population of 5.4m, representing 67% of the total (or 82% of the adult population), 38,359 tested positive, representing 1.06% of those tested. The government considered the mass test would significantly assist in controlling the virus and avoid a lockdown and may repeat the exercise at a later date.[217]
South Korea
South Korea's broad testing approach helped reduce spread. Testing capacity, largely in private sector labs, was built up over several years by the South Korean government in the early 2000s.[218]
The government exploited the resident registration number (RRN) system. Authorities mobilized young men who were eligible for military service as social service agents, security and public health doctors. Public health doctors were mainly dispatched to public health centers and life treatment centers where mildly ill patients were accommodated. They performed PCR tests and managed mild patients. Social service agents worked in pharmacies to fill staff shortages. Korea's 10k PCR tests per million residents was the world's highest as of 13 April rising to 20k by mid-June. Twenty-seven Korean companies exported test kits worth $48.6 million in March, and were asked to provide test kits or humanitarian assistance by more than 120 countries. Korean authorities set up a treatment center to isolate and manage patients with asymptomatic and minor illnesses in one facility in order to vacate hospital beds for the more severely ill.
Centers were sited mainly at national facilities and corporate training centers. The failure of Korea's MERS quarantine in May 2015 left Korea more prepared for COVID-19 than countries that did not face that pandemic. Then President Park Geun-hye allowed Korean CDC-approved private sector testing for infectious diseases in 2016. Korea already had a system for isolating, testing and treating infectious disease patients separately from others. Patients with respiratory illness but no epidemiological relevance were treated at the National Hospital, and those with epidemiological relevance were treated at selected clinics.[219][220][221][222][223][224][225][226][227]
Korea established a large scale drive-through/walk-through" test testing program. However, the most common method was "mobile examination". In Daegu City, 54% of samples were collected by 23 March in home or hospital. Collecting samples door-to-door of avoided the risk of travel by possibly infected patients, but required additional staff. Korea solved the problem by drafting more than 2,700 public insurance doctors.[219][223][222]
The government disclosed personal information to the public via KCDC without patient consent. The authorities used digital surveillance to trace possible spread.[220][223][224][226][227][228][229][230][231][232]
Taiwan
Health insurance IDs and national identification card numbers were used to trace contacts.[233][234][235][236]
United Arab Emirates
In January 2021, the COVID-19 testing results of the UAE came under scrutiny, as Denmark suspended the Emirati flights for five days. The European nation said that it barred the flights from the UAE due to growing suspicion of irregularities in the testing process being followed in the Gulf nation. Denmark's Minister of Transport, Benny Engelbrecht said that they were taking time to ensure that the negative tests of travelers from the Emirates were a real screening carried out appropriately.[237]
United States
New York State
New York State's control measures consisted of PCR tests, stay-at-home measures and strengthening the healthcare system. On 29 February before its first case, the state allowed testing at the Wordsworth Center. They managed to convince the CDC to approve tests at state laboratories and the FDA to approve a test kit. As of 13 March the state was conducting more than 1,000 daily tests, growing to 10,000/day on 19 March. In April, the number exceeded 20,000. Many people queued at hospitals to get tested. On 21 March New York City health officials directed medical providers to test only those entering the hospital, for lack of PPE.[226][238][239][240][241]
USS Theodore Roosevelt
Following an outbreak, 94% of the 4,800 aircraft carrier crew were tested. Roughly 60 percent of the 600-plus sailors who tested positive were asymptomatic.[242] Five infected sailors who completed quarantine subsequently developed flu-like symptoms and again tested positive.[243]
Nevada
In 2020, Nevada received a donation of 250,000 Covid testing kits, which were a product of China's leading genetics company, BGI Group. A UAE-based firm owned by Tahnoun bin Zayed Al Nahyan, Group 42 partnered with the BGI Group to supply the testing kits to Nevada. However, the US Department of Homeland Security and the State Department raised a warning for Nevada hospitals to not use the Chinese-made testing kits, as there were concerns around the involvement of the Chinese government, test accuracy and privacy of the patients.[244]
Delayed testing
A shortage of trained medical laboratory scientists, assay reagents, analyzers, transport medium, and PPE coupled with high demand had limited initially limited the availability of testing and led to significantly increased turnaround times.
Testing statistics by country
Testing strategies vary by country and over time,[245] with some countries testing very widely,[8] while others have at times focused narrowly on only testing the seriously ill.[6] The country that tests only people showing symptoms will have a higher figure for "Confirmed / tested" than the country that also tests others.[246] If two countries are alike in every respect, including which people they test, the one that tests more people will have a higher "Confirmed / population". Studies have also found that countries that test more, relative to the number of deaths, have lower estimated case fatality rates[9] and younger age distributions of cases.[11]
See also
References
- ↑ "Coronavirus Disease 2019 (COVID-19)". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 14 March 2020. Retrieved 9 June 2020.
- ↑ Kobokovich A, West R, Gronvall G. "Global Progress on COVID-19 Serology-Based Testing". Johns Hopkins Center for Health Security. Archived from the original on 9 June 2020. Retrieved 9 June 2020.
- 1 2 3 Kubina, Robert; Dziedzic, Arkadiusz (2020). "Molecular and Serological Tests for COVID-19. A Comparative Review of SARS-CoV-2 Coronavirus Laboratory and Point-of-Care Diagnostics". Diagnostics. 10 (6): 434. doi:10.3390/diagnostics10060434. PMC 7345211. PMID 32604919.
- ↑ "Test for Past Infection". U.S. Centers for Disease Control and Prevention (CDC). 2020. Archived from the original on 16 May 2020. Retrieved 19 May 2020.
Antibody blood tests, also called antibody tests, check your blood by looking for antibodies, which show if you had a previous infection with the virus. Depending on when someone was infected and the timing of the test, the test may not find antibodies in someone with a current COVID-19 infection.
- 1 2 3 Abbasi J (April 2020). "The Promise and Peril of Antibody Testing for COVID-19". JAMA. 323 (19): 1881–83. doi:10.1001/jama.2020.6170. PMID 32301958. Archived from the original on 20 April 2020. Retrieved 20 April 2020.
- 1 2 Brotschi M (7 March 2020). "Bund sucht nicht mehr alle Corona-Infizierten" [The federal government is no longer looking for all those infected with corona]. Der Bund (in Deutsch). ISSN 0774-6156. Archived from the original on 29 March 2020. Retrieved 9 June 2020.
- ↑ Van Beusekom, Mary (24 March 2020). "Italian doctors note high COVID-19 death rate, urge action". CIDRAP News. Archived from the original on 9 June 2020. Retrieved 9 June 2020.
- 1 2 Otmani M (22 March 2020). "COVID-19: First results of the voluntary screening in Iceland". Nordic Life Science. Archived from the original on 29 March 2020. Retrieved 9 June 2020.
- 1 2 Ward, D. (April 2020) "Sampling Bias: Explaining Wide Variations in COVID-19 Case Fatality Rates" Archived 4 May 2020 at the Wayback Machine. WardEnvironment. doi: 10.13140/RG.2.2.24953.62564/1
- ↑ Henriques M (2 April 2020). "Coronavirus: Why death and mortality rates differ". BBC News. Archived from the original on 2 April 2020. Retrieved 9 June 2020.
- 1 2 Ward D (May 2020). Sampling Bias: Explaining Variations in Age Distributions of COVID-19 Cases. Technical Report (Report). WardEnvironment. doi:10.13140/RG.2.2.27321.19047/2.
- ↑ "Why More Younger People Are Testing Positive for COVID-19". Time. Archived from the original on 26 February 2021. Retrieved 18 August 2020.
- ↑ Mina MJ, Parker R, Larremore DB (2020). "Rethinking Covid-19 Test Sensitivity – A Strategy for Containment". The New England Journal of Medicine. 383 (22): e120. doi:10.1056/NEJMp2025631. PMID 32997903. S2CID 222158786.
- ↑ "Siouxsie Wiles & Toby Morris: What we don't know about Covid-19". The Spinoff. 6 May 2020. Archived from the original on 22 August 2020. Retrieved 6 May 2020.
- ↑ "Testing for COVID-19". U.S. Centers for Disease Control and Prevention (CDC). 20 May 2020. Archived from the original on 19 May 2020. Retrieved 20 May 2020.
Two kinds of tests are available for COVID-19: viral tests and antibody tests.
- ↑ Tanner, Tari (23 September 2020). "Finland deploys coronavirus-sniffing dogs at main airport". Associated Press. Helsinki. Archived from the original on 27 October 2020. Retrieved 28 October 2020.
- ↑ Jones, Robert; Guest, Claire; Lindsay, Steve; Kleinschmidt, Immo; Bradley, John; Dewhirst, Sarah; et al. (12 August 2020). "Could bio-detection dogs be used to limit the spread of COVID-19 by travellers?". Journal of Travel Medicine. 27 (8). doi:10.1093/jtm/taaa131. ISSN 1708-8305. PMC 7454791. PMID 32789466.
- ↑ Jendrny, Paula; Twele, Friederike; Meller, Sebastian; von Köckritz-Blickwede, Maren; Osterhaus, Albertus; Ebbers, Janek; et al. (23 July 2020). "Scent dog identification of samples from COVID-19 patients – a pilot study". BMC Infectious Diseases. 20 (1). 536. doi:10.1186/s12879-020-05281-3. ISSN 1471-2334. PMC 7376324. PMID 32703188.
- 1 2 Habibzadeh, Parham; Mofatteh, Mohammad; Silawi, Mohammad; Ghavami, Saeid; Faghihi, Mohammad Ali (17 February 2021). "Molecular diagnostic assays for COVID-19: an overview". Critical Reviews in Clinical Laboratory Sciences. 58 (6): 385–398. doi:10.1080/10408363.2021.1884640. ISSN 1549-781X. PMC 7898297. PMID 33595397.
- ↑ "RNA Extraction". AssayGenie. Archived from the original on 6 May 2020. Retrieved 7 May 2020.
- 1 2 "How is the COVID-19 Virus Detected using Real Time RT-PCR?". IAEA. 27 March 2020. Archived from the original on 1 May 2020. Retrieved 5 May 2020.
- ↑ "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- 1 2 Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (April 2009). "The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments". Clinical Chemistry. 55 (4): 611–22. doi:10.1373/clinchem.2008.112797. PMID 19246619.
- ↑ "Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis" (PDF). Clinical Science. 23 September 2005. Archived (PDF) from the original on 24 November 2020. Retrieved 5 May 2020.
- ↑ "The Basics: RT-PCR". ThermoFisher Scientific. Archived from the original on 14 April 2020. Retrieved 5 May 2020.
- ↑ Kang XP, Jiang T, Li YQ, Lin F, Liu H, Chang GH, et al. (June 2010). "A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus". Virology Journal. 7: 113. doi:10.1186/1743-422X-7-113. PMC 2892456. PMID 20515509.
- ↑ Joyce C (2002). Quantitative RT-PCR. A review of current methodologies. Methods Mol. Biol. Vol. 193. pp. 83–92. doi:10.1385/1-59259-283-X:083. ISBN 978-1-59259-283-8. PMID 12325527.
- ↑ Varkonyi-Gasic E, Hellens RP (2010). "qRT-PCR of Small RNAs". Plant Epigenetics. Methods in Molecular Biology. Vol. 631. pp. 109–22. doi:10.1007/978-1-60761-646-7_10. ISBN 978-1-60761-645-0. PMID 20204872.
- ↑ "Accelerated Emergency Use Authorization (Eua) Summary Covid-19 Rt-Pcr Test (Laboratory Corporation of America)". FDA. Archived from the original on 16 January 2021. Retrieved 3 April 2020.
- ↑ Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M (April 2010). "A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines". Methods. 50 (4): S1-5. doi:10.1016/j.ymeth.2010.01.005. PMID 20215014.
- ↑ Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Domen J, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, McInnes MD, Spijker R, Van den Bruel A (24 March 2021). "Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection". Cochrane Database Syst Rev. 3 (4): CD013705. doi:10.1002/14651858.CD013705.pub2. PMC 8078597. PMID 33760236.
- ↑ "Real-Time RT-PCR Panel for Detection 2019-nCoV". U.S. Centers for Disease Control and Prevention (CDC). 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- 1 2 3 Drosten C (26 March 2020). "Coronavirus-Update Folge 22" [Coronavirus update episode 22] (PDF). NDR. Archived (PDF) from the original on 31 March 2020. Retrieved 2 April 2020.
- 1 2 "Here's where things stand on COVID-19 tests in the U.S." ScienceNews. 17 April 2020. Archived from the original on 28 April 2020. Retrieved 6 May 2020.
- 1 2 3 Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q (April 2020). "Saliva: potential diagnostic value and transmission of 2019-nCoV". International Journal of Oral Science. 12 (1): 11. doi:10.1038/s41368-020-0080-z. PMC 7162686. PMID 32300101.
- ↑ Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. (May 2003). "Identification of a novel coronavirus in patients with severe acute respiratory syndrome". The New England Journal of Medicine. 348 (20): 1967–76. doi:10.1056/NEJMoa030747. PMID 12690091.
- ↑ Ghoshal U, Vasanth S, Tejan N (2020). "A guide to laboratory diagnosis of Corona Virus Disease-19 for the gastroenterologists". Indian Journal of Gastroenterology. 39 (3): 236–242. doi:10.1007/s12664-020-01082-3. PMC 7462729. PMID 32875524.
- ↑ "COVID-19 saliva tests: What is the benefit?". Mayo Clinic. 16 April 2020. Archived from the original on 1 May 2020. Retrieved 6 May 2020.
- 1 2 "New Rutgers Saliva Test for Coronavirus Gets FDA Approval". Rutgers.edu. 13 April 2020. Archived from the original on 30 April 2020. Retrieved 1 May 2020.
- ↑ "FDA authorizes Covid-19 saliva test for emergency use". CNN. 14 April 2020. Archived from the original on 27 April 2020. Retrieved 1 May 2020.
- ↑ Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Ko AI (2020). "Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2". The New England Journal of Medicine. 383 (13): 1283–86. doi:10.1056/NEJMc2016359. PMC 7484747. PMID 32857487. S2CID 221358482.
- ↑ Service RF (2020). "Spit shines for easier coronavirus testing". Science. 369 (6507): 1041–42. Bibcode:2020Sci...369.1041S. doi:10.1126/science.369.6507.1041. PMID 32855317. S2CID 221358939.
- ↑ "Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab". Merck Manual. 29 April 2020. Archived from the original on 28 May 2020. Retrieved 6 April 2020.
- ↑ "FDA gives emergency approval to 'game changer' COVID-19 saliva test". The Washington Times. Archived from the original on 16 August 2020. Retrieved 15 August 2020.
- ↑ "Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization to Yale School of Public Health for SalivaDirect, Which Uses a New Method of Saliva Sample Processing". U.S. Food and Drug Administration (FDA) (Press release). 15 August 2020. Archived from the original on 16 August 2020. Retrieved 6 November 2020.
- 1 2
 One or more of the preceding sentences incorporates text from this source, which is in the public domain: "Risk of False Results with the Curative SARS-Cov-2 Test for COVID-19". U.S. Food and Drug Administration (FDA). 4 January 2021. Archived from the original on 4 January 2021. Retrieved 4 January 2021.
One or more of the preceding sentences incorporates text from this source, which is in the public domain: "Risk of False Results with the Curative SARS-Cov-2 Test for COVID-19". U.S. Food and Drug Administration (FDA). 4 January 2021. Archived from the original on 4 January 2021. Retrieved 4 January 2021. - ↑ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- COVID-19 Investigation Team (June 2020). "Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States". Nature Medicine. 26 (6): 861–68. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- Young BE, Ong SW, Kalimuddin S, Low JG, Tan SY, Loh J, et al. (March 2020). "Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore". JAMA. 323 (15): 1488–94. doi:10.1001/jama.2020.3204. PMC 7054855. PMID 32125362.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. (March 2020). "SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients". The New England Journal of Medicine. 382 (12): 1177–79. doi:10.1056/NEJMc2001737. PMC 7121626. PMID 32074444.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (May 2020). "Virological assessment of hospitalized patients with COVID-2019". Nature. 581 (7809): 465–69. Bibcode:2020Natur.581..465W. doi:10.1038/s41586-020-2196-x. PMID 32235945.
- ↑ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Young et al. (2020)
- ↑ Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- COVID-19 Investigation Team (June 2020). "Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States". Nature Medicine. 26 (6): 861–68. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- ↑ Zimmer C (5 May 2020). "With Crispr, a Possible Quick Test for the Coronavirus". The New York Times. ISSN 0362-4331. Archived from the original on 14 May 2020. Retrieved 14 May 2020.
- ↑ "STOPCovid". stopcovid.science. Archived from the original on 10 June 2020. Retrieved 14 June 2020.
- ↑ Joung J, Ladha A, Saito M, Segel M, Bruneau R, Huang MW, et al. (May 2020). "Point-of-care testing for COVID-19 using SHERLOCK diagnostics". medRxiv: 2020.05.04.20091231. doi:10.1101/2020.05.04.20091231. PMC 7273289. PMID 32511521. Archived from the original on 16 May 2021. Retrieved 2 July 2021.
- 1 2 3 4 5 6 "Developing Antibodies and Antigens for COVID-19 Diagnostics". Technology Networks. 6 April 2020. Archived from the original on 30 April 2020. Retrieved 30 April 2020.
- ↑ Guglielmi G (2020). "Fast coronavirus tests: what they can and can't do". Nature. 585 (7826): 496–498. Bibcode:2020Natur.585..496G. doi:10.1038/d41586-020-02661-2. PMID 32939084. S2CID 221768935.
- ↑ "Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing". whitehouse.gov. 17 April 2020. Archived from the original on 20 January 2021. Retrieved 30 April 2020 – via National Archives.
- ↑ Müllender, Friederike (11 March 2021). "Grundschulen – Corona-Pool-Tests gelten als kindgerecht, unkompliziert und sicher" (in Deutsch). Deutschlandfunk. Archived from the original on 24 July 2021. Retrieved 5 June 2021.
- ↑ "NIH launches competition to speed COVID-19 diagnostics". AAAS. 29 April 2020. Archived from the original on 1 May 2020. Retrieved 1 May 2020.
- 1 2 "What to know about the three main types of coronavirus tests". CNN. 29 April 2020. Archived from the original on 10 May 2020. Retrieved 30 April 2020.
- 1 2 Dinnes, J.; Deeks, J. J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; Domen, J.; Dretzke, J.; Ferrante Di Ruffano, L.; Harris, I. M.; Price, M. J.; Taylor-Phillips, S.; Hooft, L.; Leeflang, M. M.; McInnes, M. D.; Spijker, R.; Van Den Bruel, A.; Cochrane COVID-19 Diagnostic Test Accuracy Group (2021). "How accurate are rapid tests for diagnosing COVID-19?". The Cochrane Database of Systematic Reviews. 3: CD013705. doi:10.1002/14651858.CD013705.pub2. PMC 8078597. PMID 33760236. Archived from the original on 8 February 2022. Retrieved 2021-12-20.
- ↑ "Rapid Tests". Rapid Tests. Archived from the original on 31 May 2021. Retrieved 2 July 2021.
- ↑ Shaw, Jonathan (3 August 2020). "Failing the Coronavirus-Testing Test". Harvard Magazine. Archived from the original on 30 June 2021. Retrieved 2 July 2021.
- 1 2 Office of the Commissioner (9 May 2020). "Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients". FDA. Archived from the original on 29 May 2021. Retrieved 2 July 2021.
- 1 2 3 4 Klasse, P. J. (2014-09-09). "Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives". Advances in Biology. Hindawi Limited. 2014: 1–24. doi:10.1155/2014/157895. ISSN 2356-6582. PMC 4835181. PMID 27099867.
- ↑ "The next frontier in coronavirus testing: Identifying the full scope of the pandemic, not just individual infections". STAT. 27 March 2020. Archived from the original on 29 June 2020. Retrieved 30 April 2020.
- 1 2 Tang, E.W. (2020). "Testing for SARS-Cov-2 (COVID-19): A General Review". Rhode Island Medical Journal. 103 (8): 26–29. PMID 32900007 – via EBSCO.
- ↑ "What Immunity to COVID-19 Really Means". Scientific American. 10 April 2020. Archived from the original on 28 April 2020.
- 1 2 Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. (June 2020). "Antibody tests for identification of current and past infection with SARS-CoV-2". The Cochrane Database of Systematic Reviews. 2020 (6): CD013652. doi:10.1002/14651858.CD013652. PMC 7387103. PMID 32584464. S2CID 220061130.
- ↑ "Cellex Emergency Use Authorization". FDA. 1 April 2020. Archived from the original on 9 April 2020. Retrieved 10 April 2020.
- ↑ "Will an Antibody Test Allow Us to Go Back to School or Work?". The New York Times. 10 April 2020. Archived from the original on 15 April 2020. Retrieved 15 April 2020.
- ↑ "Mount Sinai Emergency Use Authorization". FDA. 15 April 2020. Archived from the original on 25 April 2020. Retrieved 18 April 2020.
- ↑ Bauer G (2020). "The variability of the serological response to SARS-corona virus-2: Potential resolution of ambiguity through determination of avidity (functional affinity)". Journal of Medical Virology. 93 (1): 311–322. doi:10.1002/jmv.26262. PMC 7361859. PMID 32633840.
- ↑ Ravi N, Cortade DL, Ng E, Wang SX (2020). "Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape". Biosensors and Bioelectronics. 165: 112454. doi:10.1016/j.bios.2020.112454. PMC 7368663. PMID 32729549.
- ↑ Goudouris ES (2020). "Laboratory diagnosis of COVID-19". Jornal de Pediatria. 97 (1): 7–12. doi:10.1016/j.jped.2020.08.001. PMC 7456621. PMID 32882235.
- 1 2 3 4 "Global Progress on COVID-19 Serology-Based Testing". Johns Hopkins Center for Health Security. Archived from the original on 14 June 2020. Retrieved 14 June 2020.
- 1 2 Tan, Chee Wah; Chia, Wan Ni; Qin, Xijian; Liu, Pei; Chen, Mark I.-C.; Tiu, Charles; Hu, Zhiliang; Chen, Vivian Chih-Wei; Young, Barnaby E.; Sia, Wan Rong; Tan, Yee-Joo; Foo, Randy; Yi, Yongxiang; Lye, David C.; Anderson, Danielle E.; Wang, Lin-Fa (September 2020). "A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction". Nature Biotechnology. 38 (9): 1073–1078. doi:10.1038/s41587-020-0631-z. PMID 32704169. S2CID 220720953.
- 1 2 3 Mallapaty, Smriti (18 April 2020). "Will antibody tests for the coronavirus really change everything?". Nature. 580 (7805): 571–572. Bibcode:2020Natur.580..571M. doi:10.1038/d41586-020-01115-z. PMID 32313159. S2CID 216048544. Archived from the original on 24 June 2020. Retrieved 20 April 2020.
- 1 2 3 "Q&A on COVID-19 Antibody Tests". factcheck.org. 27 April 2020. Archived from the original on 27 April 2020. Retrieved 28 April 2020.
- ↑ "Neutralising antibody". Biology-Online. 2008. Archived from the original on 8 July 2018. Retrieved 4 July 2009.
- ↑ Schmaljohn AL (July 2013). "Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts". Current HIV Research. 11 (5): 345–53. doi:10.2174/1570162x113116660057. PMID 24191933.
- ↑ Rhorer, J.; Ambrose, C. S.; Dickinson, S.; Hamilton, H.; Oleka, N. A.; Malinoski, F. J.; Wittes, J. (24 July 2009). "Virus neutralization by antibodies". Vaccine. Virology Blog. 27 (7): 1101–1110. doi:10.1016/j.vaccine.2008.11.093. PMID 19095024. Archived from the original on 23 April 2020. Retrieved 29 April 2020.
- ↑ "expert reaction to announcement by Roche of its new serology test for COVID-19 antibodies". Science Media Centre. 17 April 2020. Archived from the original on 30 April 2020. Retrieved 28 April 2020.
- ↑ Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH (September 2007). "Disappearance of antibodies to SARS-associated coronavirus after recovery". The New England Journal of Medicine. NEJM. 357 (11): 1162–63. doi:10.1056/NEJMc070348. PMID 17855683.
- 1 2 "Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study" (PDF). Journal of Immunology. 19 April 2011. Archived (PDF) from the original on 1 May 2020. Retrieved 1 May 2020.
- ↑ Leslie M (May 2020). "T cells found in coronavirus patients 'bode well' for long-term immunity". Science. 368 (6493): 809–10. Bibcode:2020Sci...368..809L. doi:10.1126/science.368.6493.809. PMID 32439770. S2CID 218834495.
- ↑ Calvo-Henriquez, Christian; Maldonado-Alvarado, Byron; Chiesa-Estomba, Carlos; Rivero-Fernández, Irene; Sanz-Rodriguez, Marta; Villarreal, Ithzel María; Rodriguez-Iglesias, Miguel; Mariño-Sánchez, Franklin; Rivero-de-Aguilar, Alejandro; Lechien, Jerome R.; Martínez-Capoccioni, Gabriel (24 June 2020). "Ethyl alcohol threshold test: a fast, reliable and affordable olfactory Assessment tool for COVID-19 patients". European Archives of Oto-Rhino-Laryngology. 277 (10): 2783–2792. doi:10.1007/s00405-020-06131-3. ISSN 0937-4477. PMC 7312102. PMID 32583183.
- ↑ Hayes, John; Exten, Cara; State, Penn. "At-home DIY smell tests could catch Covid-19 cases". CNN Health. The Conversation. Archived from the original on 31 July 2021. Retrieved 7 September 2021.
- ↑ Menni, Cristina; Sudre, Carole H.; Steves, Claire J.; Ourselin, Sebastien; Spector, Tim D. (21 November 2020). "Widespread smell testing for COVID-19 has limited application – Authors' reply". The Lancet. 396 (10263): 1630–1631. doi:10.1016/S0140-6736(20)32316-3. ISSN 0140-6736. PMC 7832202. PMID 33157000.
- 1 2 3 Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (July 2020). "Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients". AJR. American Journal of Roentgenology. 215 (1): 87–93. doi:10.2214/AJR.20.23034. PMID 32174129.
Known features of COVID-19 on initial CT include bilateral multilobar ground-glass opacification (GGO) with a peripheral or posterior distribution, mainly in the lower lobes and less frequently within the right middle lobe.
- ↑ Manigandan S, Wu M, Pugazhendhi A, Brindhadevi K (2020). "A systematic review on recent trends in transmission, diagnosis, prevention and imaging features of COVID-19". Process Biochemistry. 98: 233–40. doi:10.1016/j.procbio.2020.08.016. PMC 7439988. PMID 32843849.
- ↑ Lee EY, Ng MY, Khong PL (April 2020). "COVID-19 pneumonia: what has CT taught us?". The Lancet. Infectious Diseases. 20 (4): 384–85. doi:10.1016/S1473-3099(20)30134-1. PMC 7128449. PMID 32105641.
- ↑ "ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection". American College of Radiology. 22 March 2020. Archived from the original on 13 May 2020. Retrieved 20 May 2020.
- ↑ "Dutch corona blood test from Eindhoven goes international". 19 April 2021. Archived from the original on 27 April 2021. Retrieved 2 July 2021.
- ↑ Bart Biesemans. "Bees in the Netherlands trained to detect COVID-19 infections". Reuters. Archived from the original on 30 June 2021. Retrieved 2 July 2021.
- ↑ Jon Henley. "Dogs can better detect Covid in humans than lateral flow tests, finds study". The Guardian. Archived from the original on 29 June 2021. Retrieved 2 July 2021.
- ↑ "Todos Medical Announces Positive Data in Hospitalized and Outpatient Setting for TolloTest™, a Novel SARS-CoV-2 3CL Protease Biomarker Assay". www.yahoo.com. Archived from the original on 1 December 2021. Retrieved 16 February 2022.
- ↑ Roser M, Ritchie H, Ortiz-Ospina E (4 March 2020). "Coronavirus Disease (COVID-19) – Statistics and Research". Our World in Data. Archived from the original on 19 March 2020. Retrieved 2 July 2021 – via ourworldindata.org.
- ↑ Schnirring, Lisa (11 January 2020). "China releases genetic data on new coronavirus, now deadly". CIDRAP. Archived from the original on 11 January 2020. Retrieved 12 January 2020.
- ↑ Shu Y, McCauley J (March 2017). "GISAID: Global initiative on sharing all influenza data – from vision to reality". Euro Surveillance. 22 (13). doi:10.2807/1560-7917.ES.2017.22.13.30494. PMC 5388101. PMID 28382917.
- ↑ Ioannidis, John P.A. (17 March 2020). "A fiasco in the making? As the coronavirus pandemic takes hold, we are making decisions without reliable data". STAT. Archived from the original on 5 April 2020. Retrieved 22 March 2020.
- ↑ "'Test, Test, Test': WHO Chief's Coronavirus Message to World". The New York Times. Reuters. 16 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- ↑ Farge E, Revill J (17 March 2020). "'Test, test, test': WHO chief's coronavirus message to world". Reuters. Archived from the original on 3 November 2020. Retrieved 6 November 2020.
- ↑ "Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK" (PDF). European Centre for Disease Prevention and Control. 25 March 2020. pp. 15–16. Archived (PDF) from the original on 26 March 2020. Retrieved 29 March 2020.
the current shortages of laboratory consumables and reagents affect diagnostic capacity and hamper the epidemic response at the national and local levels. The laboratories have experienced delayed or missing deliveries of swabbing material, plastic consumables, RNA extraction and RT-PCR reagents, and PPE. This is affecting laboratories in all EU/EEA countries.
- ↑ Baird RP (24 March 2020). "Why Widespread Coronavirus Testing Isn't Coming Anytime Soon". The New Yorker. Archived from the original on 28 March 2020. Retrieved 29 March 2020.
South Dakota, said that her state's public-health laboratory—the only lab doing COVID-19 testing in the state—had so much trouble securing reagents that it was forced to temporarily stop testing altogether. also noted critical shortages of extraction kits, reagents, and test kits
- ↑ Ossola A (25 March 2020). "Here are the coronavirus testing materials that are in short supply in the US". Quartz. Archived from the original on 26 March 2020. Retrieved 29 March 2020.
extract the virus's genetic material—in this case, RNA—using a set of chemicals that usually come in pre-assembled kits. 'The big shortage is extraction kits' There are no easy replacements here: 'These reagents that are used in extraction are fairly complex chemicals. They have to be very pure, and they have to be in pure solution'
- ↑ Temple-Raston, Dina (6 November 2020). "CDC Report: Officials Knew Coronavirus Test Was Flawed But Released It Anyway". NPR. Archived from the original on 11 June 2021. Retrieved 20 March 2021.
- ↑ Armario, Christine (7 October 2020). "Peru bet heavily on cheap COVID tests; it didn't go well". Associated Press. Archived from the original on 14 January 2021. Retrieved 20 March 2021.
- ↑ jkiger@postbulletin.com, Jeff Kiger (12 March 2020). "Mayo Clinic starts drive-thru testing for COVID-19". PostBulletin.com. Archived from the original on 12 March 2020. Retrieved 13 March 2020.
- ↑ Hawkins, Andrew J. (11 March 2020). "Some states are offering drive-thru coronavirus testing". The Verge. Archived from the original on 11 March 2020. Retrieved 13 March 2020.
- ↑ "South Korea's Drive-Through Testing For Coronavirus Is Fast – And Free". npr. 11 March 2020. Archived from the original on 20 March 2020. Retrieved 16 March 2020.
- ↑ Beaubien, Jason (23 February 2020). "In Age of COVID-19, Hong Kong Innovates To Test And Quarantine Thousands". NPR. Archived from the original on 24 February 2020. Retrieved 26 February 2020.
- ↑ "Pooling method allows dozens of COVID-19 tests to run simultaneously". medicalxpress.com. Archived from the original on 22 March 2020. Retrieved 24 March 2020.
- ↑ "Israeli team has coronavirus test kit to test dozens of people at once". The Jerusalem Post | JPost.com. Archived from the original on 23 March 2020. Retrieved 24 March 2020.
- ↑ Israel21c Staff (19 March 2020). "Israelis introduce method for accelerated COVID-19 testing". Israel21c. Archived from the original on 22 March 2020. Retrieved 24 March 2020.
- ↑ "We 'pool' coronavirus samples to test 1,000s at a go; we've done 30,000 since Sunday – Noguchi". GhanaWeb. 22 April 2020. Archived from the original on 15 May 2020. Retrieved 22 April 2020.
- ↑ "Pooling samples boosts Ghana's COVID-19 testing". WHO Africa. 31 July 2020. Archived from the original on 5 August 2020. Retrieved 31 July 2020.
- ↑ "Pooling samples boosts Ghana's COVID-19 testing". World Health Organization. 30 July 2020. Archived from the original on 21 August 2020. Retrieved 30 July 2020.
- ↑ "[Coronavirus] Verified 'sample pooling' introduced to prevent herd infection in S. Korea". ajudaily.com. 9 April 2020. Archived from the original on 10 April 2020. Retrieved 19 April 2020.
- ↑ "Gov. Ricketts provides update on coronavirus testing". KMTV. 24 March 2020. Archived from the original on 20 April 2020. Retrieved 19 April 2020.
- ↑ May 2020, Nicoletta Lanese (28 May 2020). "Wuhan tested millions of people for COVID-19 in just days. Could US cities do the same?". livescience.com. Archived from the original on 28 June 2020. Retrieved 28 June 2020.
- ↑ "Latest coronavirus update: UP to begin 'pool testing' of Covid suspects". Free Press Journal. Archived from the original on 17 April 2020. Retrieved 19 April 2020.
- ↑ Sumati Yengkhom. "West Bengal to start pool testing of samples in low-risk zones". The Times of India. Archived from the original on 20 April 2020. Retrieved 19 April 2020.
- ↑ "Punjab launches pool testing". Archived from the original on 4 May 2020. Retrieved 19 April 2020.
- ↑ "'Chhattisgarh to adopt pool sample testing': Health minister TS Singh Deo on Covid-19". Hindustan Times. 15 April 2020. Archived from the original on 19 April 2020. Retrieved 19 April 2020.
- ↑ "Maharashtra to go for pool testing to defeat coronavirus". Deccan Herald. 12 April 2020. Archived from the original on 15 April 2020. Retrieved 19 April 2020.
- ↑ "Origami Assays". Origami Assays. 2 April 2020. Archived from the original on 5 April 2020. Retrieved 7 April 2020.
- ↑ Pulia MS, O'Brien TP, Hou PC, Schuman A, Sambursky R (August 2020). "Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic". Annals of Medicine. 52 (5): 207–14. doi:10.1080/07853890.2020.1763449. PMC 7877955. PMID 32370561. S2CID 218519851.
- ↑ "Which States Are Doing Enough Testing? This Benchmark Helps Settle The Debate". NPR.org. 22 April 2020. Archived from the original on 11 May 2020. Retrieved 11 May 2020.
- ↑ Lee, Timothy B. (2 April 2020). "America's COVID-19 testing has stalled, and that's a big problem". Ars Technica. Archived from the original on 14 June 2020. Retrieved 5 April 2020.
- 1 2 3 4 Romer P. "Roadmap to responsibly reopen America" (PDF). Archived (PDF) from the original on 11 May 2020. Retrieved 11 May 2020.
- ↑ "ROADMAP TO PANDEMIC RESILIENCE" (PDF). Edmond J. Safra Center for Ethics. 20 April 2020. Archived (PDF) from the original on 20 May 2020. Retrieved 19 May 2020.
- ↑ "Certified Service Providers". Pacific Biosciences. Archived from the original on 10 June 2020. Retrieved 18 May 2020.
- ↑ "Service Provider Program – US". www.thermofisher.com. ThermoFisher Scientific. Archived from the original on 10 June 2020. Retrieved 18 May 2020.
- ↑ "Paul Romer". paulromer.net. Simulating Covid-19: Part 2. Archived from the original on 18 May 2020. Retrieved 19 May 2020.
- ↑ Lewis, Tanya. "Slovakia Offers a Lesson in How Rapid Testing Can Fight COVID". Scientific American. Archived from the original on 19 April 2021. Retrieved 19 April 2021.
- ↑ Pavelka, Martin; Van-Zandvoort, Kevin; Abbott, Sam; Sherratt, Katharine; Majdan, Marek; Group5, CMMID COVID-19 working; Analýz, Inštitút Zdravotných; Jarčuška, Pavol; Krajčí, Marek; Flasche, Stefan; Funk, Sebastian (23 March 2021). "The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia". Science. 372 (6542): 635–641. Bibcode:2021Sci...372..635P. doi:10.1126/science.abf9648. ISSN 0036-8075. PMC 8139426. PMID 33758017.
- ↑ "Slovakia's mass Covid testing cut infection rate by 60%, researchers say". The Guardian. 7 December 2020. Archived from the original on 5 May 2021. Retrieved 30 April 2021.
- ↑ Sharif S, Ikram A, et al. (24 June 2020). "Detection of SARs-CoV-2 in wastewater, using the existing environmental surveillance network: An epidemiological gateway to an early warning for COVID-19 in communities". medRxiv. doi:10.1101/2020.06.03.20121426. S2CID 219322544. Archived from the original on 27 September 2020. Retrieved 16 February 2022.
- ↑ "Coronavirus traces found in March 2019 sewage sample, Spanish study shows". Reuters. 26 June 2020. Archived from the original on 21 August 2021. Retrieved 28 July 2021.
- ↑ Kreier, Freda (10 May 2021). "The myriad ways sewage surveillance is helping fight COVID around the world". Nature. doi:10.1038/d41586-021-01234-1. PMID 33972790. S2CID 234360319. Archived from the original on 28 July 2021. Retrieved 28 July 2021.
- ↑ Agrawal, Shelesh; Orschler, Laura; Lackner, Susanne (December 2021). "Long-term monitoring of SARS-CoV-2 RNA in wastewater of the Frankfurt metropolitan area in Southern Germany". Scientific Reports. 11 (1): 5372. Bibcode:2021NatSR..11.5372A. doi:10.1038/s41598-021-84914-2. PMC 7940401. PMID 33686189.
- ↑ Rooney, Christopher M.; Moura, Ines B.; Wilcox, Mark H. (January 2021). "Tracking COVID-19 via sewage". Current Opinion in Gastroenterology. 37 (1): 4–8. doi:10.1097/MOG.0000000000000692. ISSN 0267-1379. PMID 33074996. S2CID 224811450.
- ↑ Larsen, David A.; Wigginton, Krista R. (October 2020). "Tracking COVID-19 with wastewater". Nature Biotechnology. 38 (10): 1151–1153. doi:10.1038/s41587-020-0690-1. ISSN 1546-1696. PMC 7505213. PMID 32958959.
- ↑ Michael-Kordatou, I.; Karaolia, P.; Fatta-Kassinos, D. (October 2020). "Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification". Journal of Environmental Chemical Engineering. 8 (5): 104306. doi:10.1016/j.jece.2020.104306. PMC 7384408. PMID 32834990.
- ↑ Seeger, Cordula. "Abwasserbasierte EpidemiologieAbwassermonitoring als Frühwarnsystem für Pandemien" (PDF). Archived (PDF) from the original on 21 August 2021. Retrieved 28 July 2021.
- ↑ "[New Product] COVID-19 Kit". kogene.co.kr. 27 February 2020. Archived from the original on 23 April 2020.
- ↑ "Letter from FDA". FDA. 27 March 2020. Archived from the original on 28 March 2020. Retrieved 2 April 2020.
- 1 2 ID NOW COVID-19 Archived 16 January 2021 at the Wayback Machine, Instruction for Use, FDA
- ↑ "The scramble for the rapid coronavirus tests everybody wants". The Washington Post. 1 April 2020. Archived from the original on 10 February 2021. Retrieved 2 July 2021.
- 1 2 "FDA issues emergency approval of new antigen test that is cheaper, faster and simpler". The Washington Post. 9 May 2020. Archived from the original on 26 January 2021. Retrieved 2 July 2021.
- 1 2 3 Sofia 2 SARS Antigen FIA Archived 2 April 2021 at the Wayback Machine Instructions for Use, FDA.gov
- 1 2 3 Mark Peplow (14 June 2021). "COVID-19 test used in UK mass screening program receives stinging rebuke from FDA". Archived from the original on 15 June 2021. Retrieved 2 July 2021.
- ↑ FDA Division of Industry and Consumer Education (10 June 2021). "Stop Using Innova Medical Group SARS-CoV-2 Antigen Rapid Qualitative Test: FDA Safety Communication". FDA. Archived from the original on 2 July 2021. Retrieved 2 July 2021.
- ↑ Michael J. Mina; Tim E. Peto; Marta García-Fiñana; Malcolm G. Semple; Iain E Buchan (17 February 2021). "Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19". The Lancet. 397 (10283): 1425–27. doi:10.1016/S0140-6736(21)00425-6. PMC 8049601. PMID 33609444.
- ↑ "NIH Begins Study to Quantify Undetected Cases of Coronavirus Infection | NIH: National Institute of Allergy and Infectious Diseases". niaid.nih.gov. Archived from the original on 10 April 2020. Retrieved 11 April 2020.
- ↑ Mandavilli A, Thomas K (10 April 2020). "Will an Antibody Test Allow Us to Go Back to School or Work?". The New York Times. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ↑ "Quest Diagnostics Launches Consumer-Initiated COVID-19 Antibody Test Through QuestDirect™". Quest Diagnosics. 28 April 2020. Archived from the original on 17 May 2021. Retrieved 2 July 2021.
- ↑ Fellmann F. (March 2020). (in German) "Jetzt beginnt die Suche nach den Genesenen" Archived 28 March 2020 at the Wayback Machine. Tages Anzeiger. Retrieved 28 March 2020.
- ↑ Herrera, Tim (27 October 2020). "What You Need to Know About the Covid-19 Antibody Test". The New York Times. Archived from the original on 1 July 2021. Retrieved 18 July 2021.
- ↑ "Quotient Limited". quotientbd.com. Archived from the original on 3 May 2020. Retrieved 10 May 2020.
- ↑ "Quotient Limited Receives CE Mark for Its SARS-CoV-2 (COVID-19) Antibody Microarray". HospiMedica. 4 May 2020. Archived from the original on 10 May 2020. Retrieved 6 May 2020.
- ↑ "Quotient Limited Gets FDA Emergency Use Authorization for Coronavirus Antibody Test". 360dx.com. 360Dx. 28 September 2020. Archived from the original on 21 August 2021. Retrieved 18 July 2021.
- ↑ Strassheim, Isabel. "Antikörper-Nachweis zugelassen: Schweizer Start-up zieht bei Covid-Test mit Roche gleich" [Antibody detection approved: Swiss start-up draws level with Roche in Covid test]. Tages Anzeiger. Archived from the original on 2 July 2021. Retrieved 25 May 2020.
- ↑ "EUA Authorized Serology Test Performance". U.S. Food and Drug Administration (FDA). 7 May 2020. Archived from the original on 8 May 2020. Retrieved 8 May 2020.
- ↑ Bastos, Mayara Lisboa; Tavaziva, Gamuchirai; Abidi, Syed Kunal; Campbell, Jonathon R.; et al. (1 July 2020). "Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis". BMJ. 370: m2516. doi:10.1136/bmj.m2516. ISSN 1756-1833. PMC 7327913. PMID 32611558.
- ↑ Spencer, Elizabeth; Henighan, Carl (1 September 2020). "Overview of BMJ: Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis". CEBM. Archived from the original on 3 October 2020. Retrieved 24 September 2020.
- ↑ Spencer, Elizabeth; Jefferson, Tom; Brassey, Jon; Heneghan, Carl (11 September 2020). "When is Covid, Covid?". CEBM. Archived from the original on 19 September 2020. Retrieved 19 September 2020.
- ↑ Jefferson, Tom; Spencer, Elizabeth; Brassey, Jon; Heneghan, Carl (3 September 2020). "Viral cultures for COVID-19 infectivity assessment. Systematic review". medRxiv. doi:10.1101/2020.08.04.20167932. S2CID 220962177. Archived from the original on 18 January 2021. Retrieved 2 July 2021.
- ↑ Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (March 2020). "Detection of SARS-CoV-2 in Different Types of Clinical Specimens". JAMA. 323 (18): 1843–44. doi:10.1001/jama.2020.3786. PMC 7066521. PMID 32159775.
- 1 2 Ferran, Maureen (7 May 2020). "COVID-19 tests are far from perfect, but accuracy isn't the biggest problem". Popular Science. Archived from the original on 11 May 2020. Retrieved 10 May 2020.
- ↑ "Serological testing for SARS-CoV-2 antibodies". American Medical Association. 14 May 2020. Archived from the original on 28 May 2020. Retrieved 29 May 2020.
- ↑ "Interim Guidelines for COVID-19 Antibody Testing". U.S. Centers for Disease Control and Prevention (CDC). 23 May 2020. Archived from the original on 29 May 2020. Retrieved 29 May 2020.
- ↑ Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J (August 2020). "Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure". Annals of Internal Medicine. 173 (4): 262–67. doi:10.7326/M20-1495. PMC 7240870. PMID 32422057.
- ↑ "RT-PCR Testing". www.idsociety.org. Archived from the original on 24 June 2021. Retrieved 16 February 2021.
- ↑ Böger, Beatriz; Fachi, Mariana M.; Vilhena, Raquel O.; Cobre, Alexandre F.; Tonin, Fernanda S.; Pontarolo, Roberto (January 2021). "Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19". American Journal of Infection Control. 49 (1): 21–29. doi:10.1016/j.ajic.2020.07.011. PMC 7350782. PMID 32659413.
- ↑ "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19". U.S. Centers for Disease Control and Prevention (CDC). 30 April 2020. Archived from the original on 6 June 2021. Retrieved 28 August 2021.
- ↑ Xiao AT, Tong YX, Zhang S (April 2020). "Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients". Clinical Infectious Diseases. 71 (16): 2249–2251. doi:10.1093/cid/ciaa460. PMC 7188124. PMID 32306036.
- 1 2 3 Engelmann I, Alidjinou EK, Ogiez J, Szunerits S (2021). "Preanalytical Issues and Cycle Threshold Values in SARS-CoV-2 Real-Time RT-PCR Testing: Should Test Results Include These?". ACS Omega. 6 (10): 6528–6536. doi:10.1021/acsomega.1c00166. PMC 7970463. PMID 33748564.
- ↑ Fauci, Dr. Anthony (July 16, 2020). "This Week in Virology". YouTube. 4:20. Archived from the original on 21 February 2022. Retrieved 16 February 2022.
- ↑ Mandavilli, Apoorva (2020-08-29). "Your Coronavirus Test Is Positive. Maybe It Shouldn't Be". The New York Times. ISSN 0362-4331. Archived from the original on 18 February 2022. Retrieved 2021-08-30.
- ↑ US CDC (July 20, 2021). "Real-Time RT-PCR Diagnostic Panel: Instructions for Use". Food and Drug Administration. p. 35. Archived from the original on 15 February 2022. Retrieved August 30, 2021.
- 1 2 van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, et al. (July 2020). "Comparison of seven commercial RT-PCR diagnostic kits for COVID-19". Journal of Clinical Virology. 128: 104412. doi:10.1016/j.jcv.2020.104412. PMC 7206434. PMID 32416600.
- ↑ "Chinese Covid-19 test kit outstrips alternatives in Dutch study". South China Morning Post. 20 May 2020. Archived from the original on 23 May 2020. Retrieved 23 May 2020.
- ↑ Heneghan, Carl; Jefferson, Tom (1 September 2020). "Virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR". CEBM. Archived from the original on 18 June 2021. Retrieved 19 September 2020.
- ↑ Lu, Jing; Peng, Jinju; Xiong, Qianling; Liu, Zhe; et al. (31 July 2020). "Clinical, immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR". EBioMedicine. 59: 102960. doi:10.1016/j.ebiom.2020.102960. PMC 7444471. PMID 32853988.
- ↑ Spencer, Elizabeth; Jefferson, Tom; Brassey, Jon; Heneghan, Carl (11 September 2020). "When is Covid, Covid?". The Centre for Evidence-Based Medicine. Archived from the original on 18 June 2021. Retrieved 19 September 2020.
- ↑ "SARS-CoV-2 RNA testing: assurance of positive results during periods of low prevalence". GOV.UK. Archived from the original on 6 May 2021. Retrieved 19 September 2020.
- ↑ "Study Raises Questions About False Negatives From Quick COVID-19 Test". NPR. 21 April 2020. Archived from the original on 1 May 2020. Retrieved 1 May 2020.
- ↑ Thomas, Katie (13 May 2020). "Coronavirus Testing Used by the White House Could Miss Infections". The New York Times. ISSN 0362-4331. Archived from the original on 13 May 2020. Retrieved 14 May 2020.
- ↑ "National laboratories". who.int. Archived from the original on 31 January 2020. Retrieved 2 March 2020.
- ↑ "PHE novel coronavirus diagnostic test rolled out across UK". GOV.UK. Archived from the original on 7 February 2020. Retrieved 12 April 2020.
In addition to processing samples from suspected cases in this country, PHE is now working as a reference laboratory for WHO, testing samples from countries that do not have assured testing capabilities.
- ↑ "Specimen referral for COVID-19 – operational details of WHO reference laboratories providing confirmatory testing for COVID-19" (PDF). World Health Organization. Archived (PDF) from the original on 5 March 2020. Retrieved 29 March 2020.
- ↑ "COVID-19: First results of the voluntary screening in Iceland". Nordic Life Science. 27 March 2020. Archived from the original on 29 March 2020. Retrieved 5 April 2020.
- ↑ "How an experiment helped one Italian town find 'submerged infections,' cut new COVID-19 cases to zero". Nationalpost. 19 March 2020. Archived from the original on 17 May 2020. Retrieved 29 March 2020.
- 1 2 3 "PCR拡充が必要 専門家会議が会見 (全文1)" [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in 日本語). Yahoo!ニュース. 5 May 2020. p. 5. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- 1 2 3 4 5 "「新型コロナウイルス感染拡大阻止 最前線からの報告" [Report from the front line to prevent the spread of new coronavirus infection]. NHK (in 日本語). 15 April 2020. Archived from the original on 19 April 2020. Retrieved 27 May 2020.
- 1 2 3 "Did Japan Just Beat the Virus Without Lockdowns or Mass Testing?". Bloomberg.com. 23 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ↑ "PCR拡充が必要 専門家会議が会見 (全文1)" [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in 日本語). Yahoo!ニュース. 5 May 2020. p. 3. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- 1 2 "新型コロナウイルス 感染爆発をどう防ぐか" [How to prevent the outbreak of new coronavirus infection]. NHK (in 日本語). 8 April 2020. Archived from the original on 8 April 2020. Retrieved 27 May 2020.
- ↑ "第1波は終息するも欧米からの帰国者経由の第2波が拡大" [The first wave is over, but the second wave is expanding via returnees from Europe and the United States]. 日経メディカル (Nikkei Medical) (in 日本語). 12 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- 1 2 "専門家に聞く"新型コロナウイルス"との闘い方と対策" [Ask experts how to fight the "new coronavirus" and countermeasures]. NHK (in 日本語). 27 March 2020. Archived from the original on 8 April 2020. Retrieved 27 May 2020.
- ↑ "新型コロナ抗原検査キット、13日から実用化 加藤厚労相が発表 PCRとの併用を想定" [New corona antigen test kit put into practical use from 13th. Minister of Health, Labor and Welfare Kato announced that it will be used in combination with PCR]. 毎日新聞 (Mainichi newspaper ) (in 日本語). 12 May 2020. Archived from the original on 27 May 2020. Retrieved 27 May 2020.
- ↑ "コロナ抗原検査が使用可能に、陽性のみ確定診断" [Corona antigen test available, positive only definitive diagnosis]. 日経メディカル (Nikkei Medical) (in 日本語). 12 May 2020. Archived from the original on 21 May 2020. Retrieved 15 May 2020.
- 1 2 "PCR拡充が必要 専門家会議が会見 (全文1)" [PCR expansion required Expert meeting (Full text 1)]. THE PAGE (in 日本語). Yahoo!ニュース. 5 May 2020. p. 4. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- 1 2 "クルーズ船112人治療で「院内感染」ゼロ!「自衛隊中央病院」はなぜ奇跡を起こせたのか" [No "nosocomial infection" with treatment of 112 cruise ships! Why did "Self-Defense Forces Central Hospital" cause a miracle?]. 週刊新潮 (Shukan Shincho) (in 日本語). 30 April 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ↑ "「PCR検査数少ないが、死亡者数・率低い」専門家会議" ["The number of PCR tests is small, but the number of deaths and rate is low" Expert meeting]. m3.com (in 日本語). 5 May 2020. Archived from the original on 8 June 2020. Retrieved 27 May 2020.
- ↑ "調査報告クルーズ船 ウイルス対策のカギは?" [Survey Report What is the key to anti-virus measures for cruise ships?]. NHK (in 日本語). 7 May 2020. Archived from the original on 12 May 2020. Retrieved 24 May 2020.
- ↑ "新型コロナウイルス感染症の現在の状況と厚生労働省の対応について(令和2年7月20日版)" [Current status of new coronavirus infection and response by the Ministry of Health, Labor and Welfare (Reiwa 20 July, 2nd edition)] (in 日本語). 厚生労働省. 20 July 2000. Archived from the original on 4 August 2020. Retrieved 1 August 2020.
- ↑ "PCR検査能力、4月の3倍 それでも受けにくいわけは" [PCR test capacity, 3 times that of April]. Asahi Shimbun (in 日本語). 28 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ↑ "日本のコロナ検査能力、米英の1割どまり" [Japan's corona inspection ability, only 10% of the US and UK] (in 日本語). The Nikkei. 21 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ↑ "新型コロナが弱毒化しているという根拠はない" [There is no evidence that the new corona is attenuated] (in 日本語). Yahoo!ニュース. 26 July 2020. Archived from the original on 27 July 2020. Retrieved 1 August 2020.
- ↑ "軽症者施設、23都府県で不足 コロナ第2波推計" [Facility for mildly ill people, Insufficient in 23 prefectures Corona second wave estimation] (in 日本語). The Nikkei. 21 July 2020. Archived from the original on 31 July 2020. Retrieved 1 August 2020.
- ↑ "患者急増、埋まりつつあるベッド 増床要請に頭抱える病院…スタッフは?一般患者は?経営は?" [The number of patients is increasing rapidly, and the beds are being filled up. Hospitals are having a request to increase the floor space ... Staff? General patients? Management?]. Mainichi Shimbun (in 日本語). 22 July 2020. Archived from the original on 29 July 2020. Retrieved 1 August 2020.
- ↑ "軽症患者ICUを圧迫 クラスターはほぼ終息 新型コロナで兵庫県対策協" [Squeezing ICU for mildly ill patients The cluster is almost over With the new corona] (in 日本語). 神戸新聞. 25 March 2020. Archived from the original on 22 October 2020. Retrieved 1 August 2020.
- ↑ "Over 3 mln COVID-19 tests conducted in Russia". TASS. 27 April 2020. Archived from the original on 11 May 2020. Retrieved 29 April 2020.
- ↑ "Popova said explosive growth in incidence was not allowed due to measures taken". TASS. 28 April 2020. Archived from the original on 29 August 2020. Retrieved 29 April 2020.
- ↑ "COVID-19 outbreak: Petition to close schools in Singapore garners 7,700 signatures to date". msn.com. Archived from the original on 29 March 2020. Retrieved 29 March 2020.
- ↑ "More than 3.6 million people tested during the weekend". The Slovak Spectator. 1 November 2020. Archived from the original on 2 January 2020. Retrieved 2 July 2021.
- ↑ Kuhn, Anthony (12 March 2020). "Experts Credit South Korea's Extensive Testing For Curbing Coronavirus Spread". NPR.org. Archived from the original on 16 March 2020. Retrieved 28 June 2020.
- 1 2 "日本が韓国の新型コロナウイルス対策から学べること──(1)検査体制" [What Japan can learn from Korea's measures against the new coronavirus ── (1) Inspection system]. Newsweek Japan (in 日本語). 2 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- 1 2 "日本が韓国の新型コロナウイルス対策から学べること──(3)情報公開" [What Japan can learn from Korea's measures against the new coronavirus ── (3) Information disclosure]. Newsweek Japan (in 日本語). 21 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ↑ "日本が韓国の新型コロナウイルス対策から学べること──(4)軽症者の隔離・管理対策:「生活治療センター」" [What Japan can learn from Korea's measures against the new coronavirus ── (4) Isolation and management measures for mildly ill people: "Life Treatment Center"]. Newsweek Japan (in 日本語). 11 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- 1 2 "韓国のコロナ対策を称える日本に欠ける視点" [Japan's lack of perspective to praise South Korea's measures against corona]. Newsweek Japan (in 日本語). 2 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- 1 2 3 "韓国式大量検査は徴兵制の賜物…新型コロナが揺さぶる「自由」の価値" [Korean-style mass inspection is a gift of conscription ... The value of "freedom" that the new corona shakes] (in 日本語). FNNプライム. 14 April 2020. Archived from the original on 27 April 2020. Retrieved 5 June 2020.
- 1 2 "韓国における新型コロナウィルス防疫事情(韓国)" [New Coronavirus Epidemic Prevention Circumstances in South Korea (Korea)] (in 日本語). 日本商工会議所. 10 May 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ↑ "韓国製PCR検査キットが新型コロナから世界を救う日" [The day when the Korean PCR test kit saves the world from the new corona]. Newsweek Japan (in 日本語). 14 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- 1 2 3 "新型ウイルス"パンデミック" 医療崩壊を防ぐには" [New virus "pandemic" How to prevent medical collapse]. NHK (in 日本語). 9 April 2020. Archived from the original on 19 April 2020. Retrieved 2 June 2020.
- 1 2 "IT活用でコロナ追跡 韓国、感染者の経路公開" [Corona tracking by utilizing IT South Korea, route disclosure of infected people]. Mainichi Shimbun (in 日本語). 16 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ↑ "コロナ対策で浮かび上がる「監視社会」韓国 個人情報をここまでさらしてよいのか" ["Surveillance society" that emerges from corona measures Can South Korea expose personal information to this extent?]. Tokyo Shimbun (in 日本語). 1 April 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ↑ "新型コロナ:「感染追跡」デジタル監視とプライバシーの新しい日常" [New Corona: "Infection Tracking" New Everyday Life in Digital Surveillance and Privacy] (in 日本語). Yahoo!ニュース. 26 March 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ↑ "韓国、コロナ隔離者に監視腕輪 「人権侵害」の声" [South Korea, Corona quarantine voice of surveillance bracelet "human rights violations"] (in 日本語). The Nikkei. 17 April 2020. Archived from the original on 29 May 2020. Retrieved 29 May 2020.
- ↑ "South Korea is watching quarantined citizens with a smartphone app". MIT Technology Review. 6 March 2020. Archived from the original on 5 June 2020. Retrieved 5 June 2020.
- ↑ "Coronavirus privacy: Are South Korea's alerts too revealing?". BBC. 5 March 2020. Archived from the original on 6 June 2020. Retrieved 5 June 2020.
- ↑ "台湾がコロナ「優等生」になった理由。閣僚に医師出身、デジタル化の一方で強まる監視" [The reason why Taiwan became a corona "honor student". A doctor from a minister, increasing surveillance while digitizing]. Business Insider (in 日本語). 1 May 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ↑ "台湾の新型コロナ対策が「善戦」しているワケ" [The reason why Taiwan's new corona measures are "good fight"]. Wedge Infinity (in 日本語). 28 February 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ↑ "台湾が新型コロナの感染拡大を抑制できている理由" [Why Taiwan is able to curb the spread of the new corona]. Wedge Infinity (in 日本語). 28 February 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ↑ "新型コロナ対応の「優等生」は「台湾・韓国・ドイツ」" [Why Taiwan is able to curb the spread of the new corona ...] (in 日本語). 日経ビジネス (Nikkei Business). 21 April 2020. Archived from the original on 8 June 2020. Retrieved 6 June 2020.
- ↑ "Covid-19: Denmark suspends flights from the Emirates". Le Figaro. Archived from the original on 2 January 2020. Retrieved 22 January 2021.
- ↑ "COVID-19 Public Policies #2 ニューヨークはいかにして検査数を増やしたのか" [COVID-19 Public Policies #2 How New York increased the number of inspections]. Office of the City of Yokohama Representative to the Americas (in 日本語). 14 May 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ↑ "Coronavirus New York: health officials provide limits on testing patients for COVID-19". Eyewitness News. 21 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ↑ "マスクも防護服も足りない! ニューヨークの病院で看護師が新型コロナウイルスに感染、死亡" [Not enough masks and protective clothing! A nurse is infected with a new coronavirus and dies at a hospital in New York]. Business Insider Japan (in 日本語). 27 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ↑ "NY州感染者数、全米2位に 感染爆発で2週間封じ込め作戦へ" [The number of infected people in New York ranks second in the United States.]. Yahoo!ニュース (in 日本語). 11 March 2020. Archived from the original on 8 June 2020. Retrieved 2 June 2020.
- ↑ "Coronavirus clue? Most cases aboard U.S. aircraft carrier are symptom-free". Reuters. 16 April 2020. Archived from the original on 11 December 2020. Retrieved 2 July 2021.
- ↑ "Sailors on sidelined USS Theodore Roosevelt get virus for second time". NBC News. Archived from the original on 21 May 2020. Retrieved 21 May 2020.
- ↑ "US warned Nevada not to use Chinese COVID tests from UAE". The Associated Press. Archived from the original on 16 October 2020. Retrieved 15 October 2020.
- ↑ "Special Report: Italy and South Korea virus outbreaks reveal disparity in deaths and tactics". Reuters. 13 March 2020. Archived from the original on 22 April 2020. Retrieved 22 June 2020.
- ↑ "Want to know how many people have the coronavirus? Test randomly". The Conversation. 13 April 2020. Archived from the original on 9 May 2020. Retrieved 7 May 2020.
 This article incorporates public domain material from the Centers for Disease Control and Prevention document: "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19".
This article incorporates public domain material from the Centers for Disease Control and Prevention document: "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19".
Further reading
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. (January 2020). "Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR". Euro Surveill. 25 (3). doi:10.2807/1560-7917.ES.2020.25.3.2000045. PMC 6988269. PMID 31992387.
- Guglielmi G (July 2020). "The explosion of new coronavirus tests that could help to end the pandemic". Nature. 583 (7817): 506–9. Bibcode:2020Natur.583..506G. doi:10.1038/d41586-020-02140-8. PMID 32681157.
- Kevadiya, Bhavesh D.; Machhi, Jatin; Herskovitz, Jonathan; Oleynikov, Maxim D.; Blomberg, Wilson R.; Bajwa, Neha; Soni, Dhruvkumar; Das, Srijanee; Hasan, Mahmudul; Patel, Milankumar; Senan, Ahmed M. (15 February 2021). "Diagnostics for SARS-CoV-2 infections". Nature Materials. 20 (5): 593–605. Bibcode:2021NatMa..20..593K. doi:10.1038/s41563-020-00906-z. ISSN 1476-4660. PMC 8264308. PMID 33589798. S2CID 231930978.
External links
| Wikiquote has quotations related to: COVID-19 testing |
- Ritchie, Hannah; Ortiz-Ospina, Esteban; Beltekian, Diana; Mathieu, Edouard; Hasell, Joe; MacDonald, Bobbie; Giattino, Charlie; Appel, Cameron; Rodés-Guirao, Lucas; Roser, Max (13 July 2020). "Coronavirus (COVID-19) Testing". Our World in Data. Archived from the original on 29 September 2020. Retrieved 16 February 2022. – International testing statistics updated twice a week.
- "COVID-19 diagnostic tech tableau". BioCentury. Archived from the original on 19 June 2020. Retrieved 22 June 2020.
- COVID-19 Testing (at least) – now Free for all? (CDC; US Congress; CSPAN video/6:00; 12 March 2020) Archived 23 March 2022 at the Wayback Machine
- "EUA Authorized Serology Test Performance". U.S. Food and Drug Administration (FDA). 25 May 2021. Archived from the original on 8 May 2020. Retrieved 16 February 2022.
- "Global Progress on COVID-19 Serology-Based Testing". Johns Hopkins Center for Health Security. Archived from the original on 12 May 2020. Retrieved 16 February 2022.
- "Testing FAQ". Johns Hopkins Coronavirus Resource Center. Archived from the original on 6 March 2022. Retrieved 16 February 2022.
- "New Rapid Testing Device for COVID-19 Immunity". Mantracourt Electronics & University of Exeter. Archived from the original on 9 November 2021. Retrieved 16 February 2022.