The Mechanism of Protein Synthesis
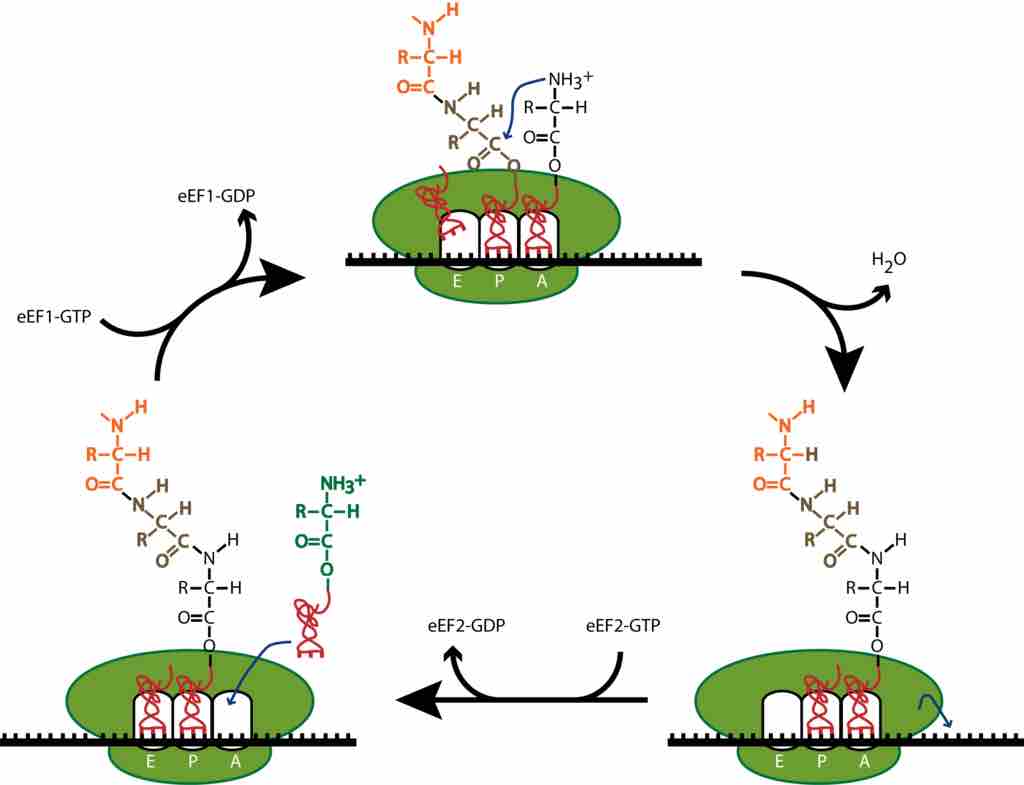
Translation elongation in eukaryotes.
During translation elongation, the incoming aminoacyl-tRNA enters the ribosome A site, where it binds if the tRNA anticodon is complementary to the A site mRNA codon. The elongation factor eEF1 assists in loading the aminoacyl-tRNA, powering the process through the hydrolysis of GTP. The growing polypeptide chain is attached to the tRNA in the ribosome P site. The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. This creates a peptide bond between the C terminus of the growing polypeptide chain and the A site amino acid. After the peptide bond is created, the growing polypeptide chain is attached to the A site tRNA, and the tRNA in the P site is empty. The ribosome translocates once codon on the mRNA. The elongation factor eEF2 assists in the translocation, powering the process through the hydrolysis of GTP. During translocation, the two tRNAs remain basepaired to their mRNA codons, so the ribosome moves over them, putting the empty tRNA in the E site (where it will be expelled from the ribosome) and the tRNA with the growing polypeptide chain in the P site. The A site moves over an empty codon, and the process repeats itself until a stop codon is reached.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: