Enthalpy
Enthalpy (H) is a measure of the total energy of a thermodynamic system. Any time a thermodynamic system undergoes a transformation or a chemical reaction, there is an energy (enthalpy) change associated with the process. Since the enthalpy of a system cannot be directly measured, we often concern ourselves with the change in enthalpy after a reaction has taken place. Any chemical reaction can be characterized by a change in enthalpy, denoted as:
Changes in Enthalpy
By absorbing heat, the temperature, and thus the enthalpy of a substance increases. The relationship is shown as:
Hess's Law
Sometimes reactions of interest take place in several steps. Hess's law addresses how to calculate the enthalpy for the overall reaction. The law states that the enthalpy change for a reaction is the same whether it occurs in one or many steps. Since enthalpy is a state function, or pathway independent, the route that the reaction takes does not change the enthalpy value. Hess's law states that the standard enthalpy change of the overall reaction is the sum of the enthalpy change of all the intermediate reactions that make up the overall reaction. For example, if you convert reactants A into products B, the overall enthalpy change will be the same whether you do it in one step or many steps.
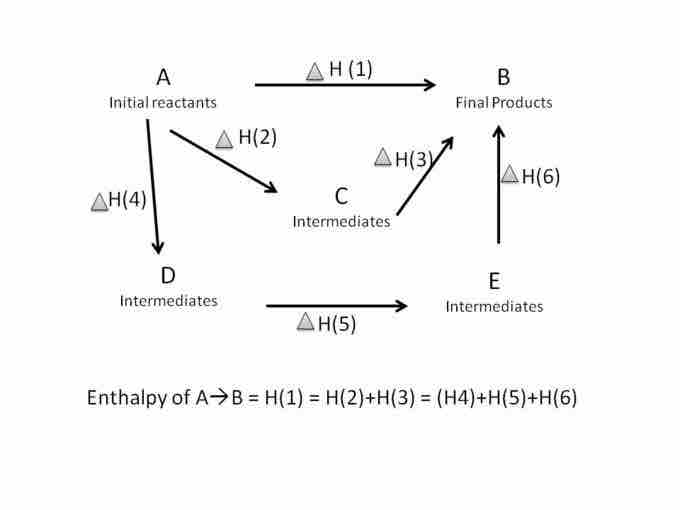
Hess's Cycle
Since enthalpy is a state function, it can be calculated in one step or in many intermediate steps.
Being able to understand changes in enthalpy is essential in understanding the nature of the overall reaction. Many reaction enthalpies have been experimentally determined and documented. Often, you will encounter an overall reaction whose enthalpy is not listed; however, you may see that the overall reaction may be split into intermediate reactions. By remembering and employing Hess's Law, the change in enthalpy for the overall reaction can be determined by adding up the enthalpies of the intermediate reactions.