Polyatomic molecules are electrically neutral groups of three or more atoms held together by covalent bonds. Molecules are distinguished from ions by their lack of electrical charge.
Molecular Chemistry and Molecular Physics
The science of molecules is called molecular chemistry or molecular physics, depending on the focus. Molecular chemistry deals with the laws governing the interaction between molecules resulting in the formation and breakage of chemical bonds; molecular physics deals with the laws governing their structure and properties. In molecular sciences, a molecule consists of a stable system (bound state) comprising two or more atoms. Molecules have fixed equilibrium geometries—bond lengths and angles—about which they continuously oscillate through vibrational and rotational motions.
A pure substance is composed of molecules with the same average geometrical structure. A molecule's chemical formula and structure are the two important factors that determine its properties, particularly reactivity.
A compound's empirical formula is the simplest integer ratio of its constitutional chemical elements. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms.
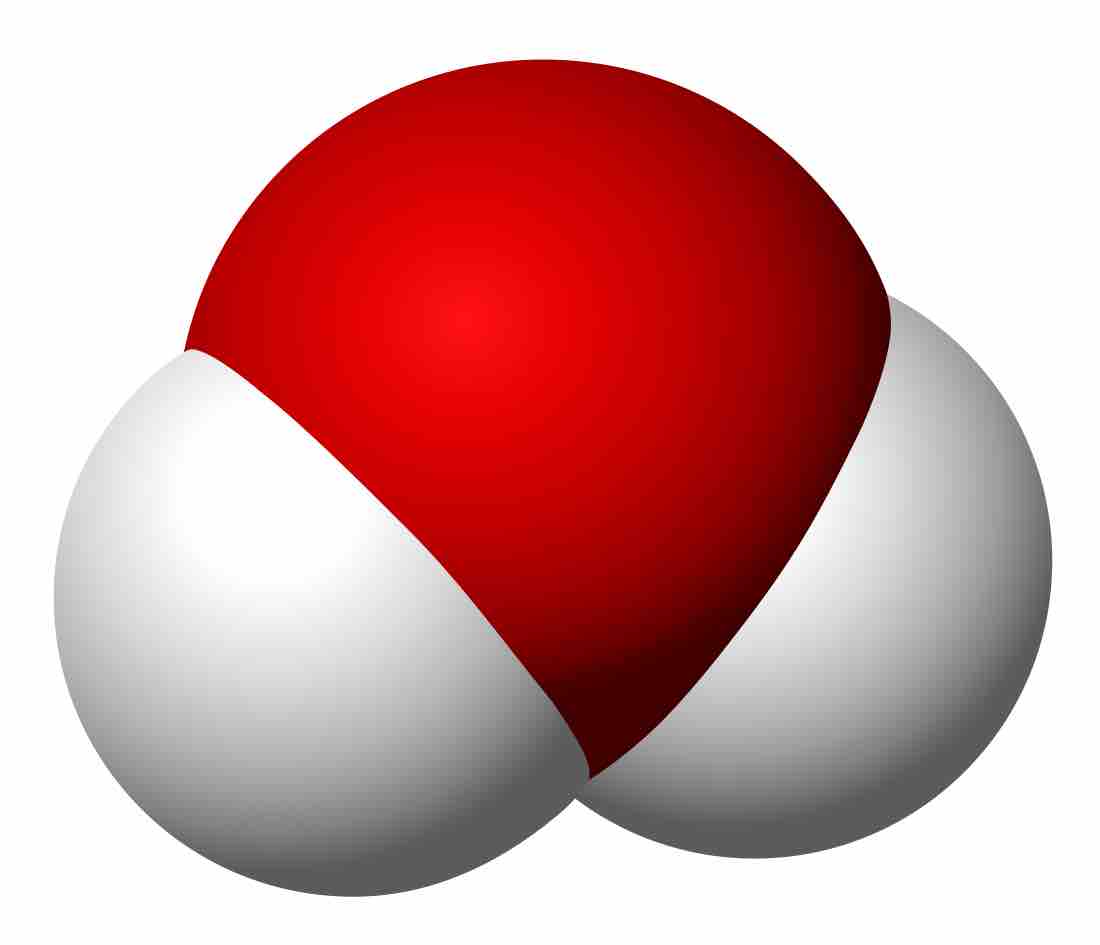
Water
Another triatomic composed of two atoms, hydrogens (white) are bound to a central oxygen (red); note that this molecule is not linear.
Ethyl alcohol, or ethanol, is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio; this does not uniquely determine the kind of molecule, however. Dimethyl ether, for example, has the same ratios as ethanol. Molecules with the same atoms in different arrangements are called isomers. For example, carbohydrates have the same ratio (carbon: hydrogen: oxygen = 1:2:1) and thus the same empirical formula, but have different total numbers of atoms in the molecule.
Molecular and Empirical Formulas
The molecular formula characterizes different molecules by reflecting their exact number of compositional atoms. Different isomers can have the same atomic composition while being different molecules, however. The empirical formula is often the same as the molecular formula, but not always; for example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.