A dipole exists when there are areas of asymmetrical positive and negative charges in a molecule. Dipole moments increase with ionic bond character and decrease with covalent bond character.
Bond dipole moment
The bond dipole moment uses the idea of the electric dipole moment to measure a chemical bond's polarity within a molecule. This occurs whenever there is a separation of positive and negative charges due to the unequal attraction that the two atoms have for the bonded electrons. The atom with larger electronegativity will have more pull for the bonded electrons than will the atom with smaller electronegativity; the greater the difference in the two electronegativities, the larger the dipole. This is the case with polar compounds like hydrogen fluoride (HF), where the atoms unequally share electron density.
Physical chemist Peter J. W. Debye was the first to extensively study molecular dipoles. Bond dipole moments are commonly measured in debyes, represented by the symbol D.
Molecules with only two atoms contain only one (single or multiple) bond, so the bond dipole moment is the molecular dipole moment. They range in value from 0 to 11 D. At one extreme, a symmetrical molecule such as chlorine, Cl2, has 0 dipole moment. This is the case when both atoms' electronegativity is the same. At the other extreme, the highly ionic gas phase potassium bromide, KBr, has a dipole moment of 10.5 D.
Bond Symmetry
Symmetry is another factor in determining if a molecule has a dipole moment. For example, a molecule of carbon dioxide has two carbon—oxygen bonds that are polar due to the electronegativity difference between the carbon and oxygen atoms. However, the bonds are on exact opposite sides of the central atom, the charges cancel out. As a result, carbon dioxide is a nonpolar molecule.
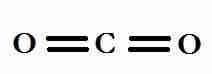
The linear structure of carbon dioxide.
The two carbon to oxygen bonds are polar, but they are 180° apart from each other and will cancel.
Molecular Dipole Moment
When a molecule consists of more than two atoms, more than one bond is holding the molecule together. To calculate the dipole for the entire molecule, add all the individual dipoles of the individual bonds as their vector. Dipole moment values can be experimentally obtained by measuring the dielectric constant. Some typical gas phase values in debye units include:
- carbon dioxide: 0 (despite having two polar C=O bonds, the two are pointed in geometrically opposite directions, canceling each other out and resulting in a molecule with no net dipole moment)
- carbon monoxide: 0.112 D
- ozone: 0.53 D
- phosgene: 1.17 D
- water vapor: 1.85 D
- hydrogen cyanide: 2.98 D
- cyanamide: 4.27 D
- potassium bromide: 10.41 D
KBr has one of the highest dipole moments because of the significant difference in electronegativity between potassium and bromine.