Concept
Version 15
Created by Boundless
Explanation of Valence Bond Theory
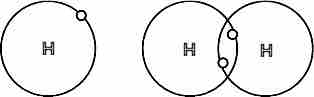
Covalent bond between hydrogen atoms
Each hydrogen atom has one electron. To complete their valence shells, they bond and share one electron with each other. This allows electrons to move about both atoms and gives both atoms access to two electrons; they become a stable H2 molecule joined by a single covalent bond.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"General Chemistry/Covalent bonds."
http://en.wikibooks.org/wiki/General_Chemistry/Covalent_bonds%23The_Valence_Bond_Model
Wikibooks
CC BY-SA 3.0.