Concept
Version 16
Created by Boundless
sp2 Hybridization
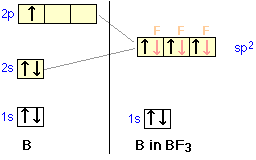
Boron configuration diagram
One of the three boron electrons is unpaired in its ground state. The atomic s- and p-orbitals in boron's outer shell mix to form three equivalent hybrid orbitals. These particular orbitals are called sp2 hybrids, meaning that this set of orbitals derives from one s- orbital and two p-orbitals of the free atom.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Hybrid orbitals - 1."
http://www.chem1.com/acad/webtext/chembond/cb06.html#SEC1
Steve Lower
CC BY-SA.