Concept
Version 16
Created by Boundless
sp2 Hybridization
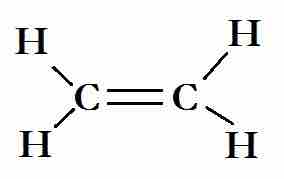
The Lewis structure for ethene
The carbon atoms are sp2 hybridized. Two sp2 hybrids bond with the hydrogen atoms, and the other forms a sigma bond with the other carbon atom. The p-orbitals that are unused by the carbon atoms in the hybridization overlap to form the C=C.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: