In a tetravalent molecule, four outer atoms are bonded to a central atom. Perhaps the most common and important example of this bond type is methane, CH4. In the ground state of the free carbon atom, there are two unpaired electrons in separate 2p orbitals. To form four bonds, the atom must have four unpaired electrons; this requires that carbon's valence 2s and 2p orbitals each contain an electron for bonding.
The single 2s orbital is spherical, different from the dumbbell-shaped 2p orbitals. This would indicate that one of the four bonds differs from the other three, but scientific tests have proven that all four bonds have equal length and energy; this is due to the hybridization of carbon's 2s and 2p valence orbitals.
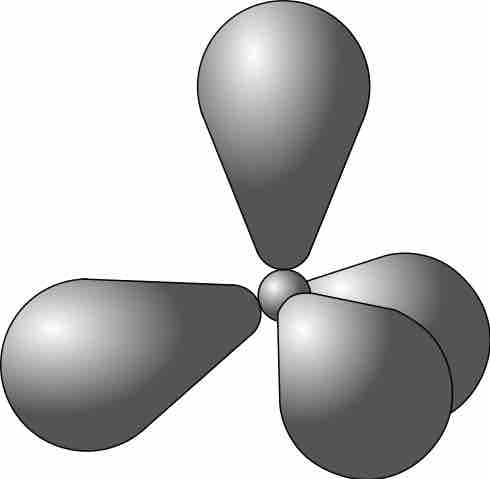
Methane
The methane molecule has four equal bonds.
In hybridization, carbon's 2s and three 2p orbitals combine into four identical orbitals, now called sp3 hybrids.
The bonds between carbon and hydrogen can form the backbone of very complicated and extensive chain hydrocarbon molecules. The simplest of these is ethane (C2H6), in which an sp3 orbital on each of the two carbon atoms joins (overlaps) to form a carbon-carbon bond; then, the remaining carbon sp3 orbital overlaps with six hydrogen 1s orbitals to form the ethane molecule.
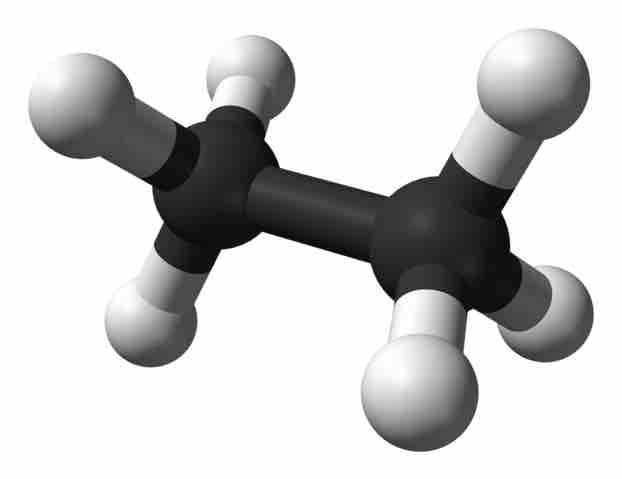
Ethane
Ethane can form by replacing one of the hydrogen atoms in CH4 with another sp3 hybridized carbon fragment.
If lone electron pairs are present on the central atom, thet can occupy one or more of the sp3 orbitals. For example, in the ammonia molecule, the fourth of the sp3 hybrid orbitals on the nitrogen contains the two remaining outer-shell electrons, which form a non-bonding lone pair.
In the water molecule, the oxygen atom can form four sp3 orbitals. Two of these are occupied by the two lone pairs on the oxygen atom, while the other two are used for bonding. The observed H-O-H bond angle in water (104.5°) is less than the tetrahedral angle (109.5°); one explanation for this is that the non-bonding electrons tend to remain closer to the central atom and thus exert greater repulsion on the other orbitals, pushing the two bonding orbitals closer together.