Chapter 25
Chemistry and the Real World
By Boundless

The Earth's atmosphere is a layer of mixed gases that is trapped near the surface due to gravitational forces.

Aurora borealis and australis are glowing sky phenomena that occur when charged particles from the sun excite atmospheric molecules.
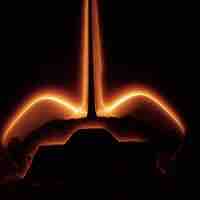
The glow observed as a space shuttle re-enters the atmosphere is due to excited NO2 releasing light to return to its ground state.
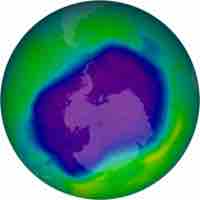
Chlorofluorocarbons have disrupted stratospheric ozone generation, resulting in a thinning of the ozone layer at the poles.

Volcanoes can cause environmental damage due to the hot lava, ash, and gases that are released during an eruption.
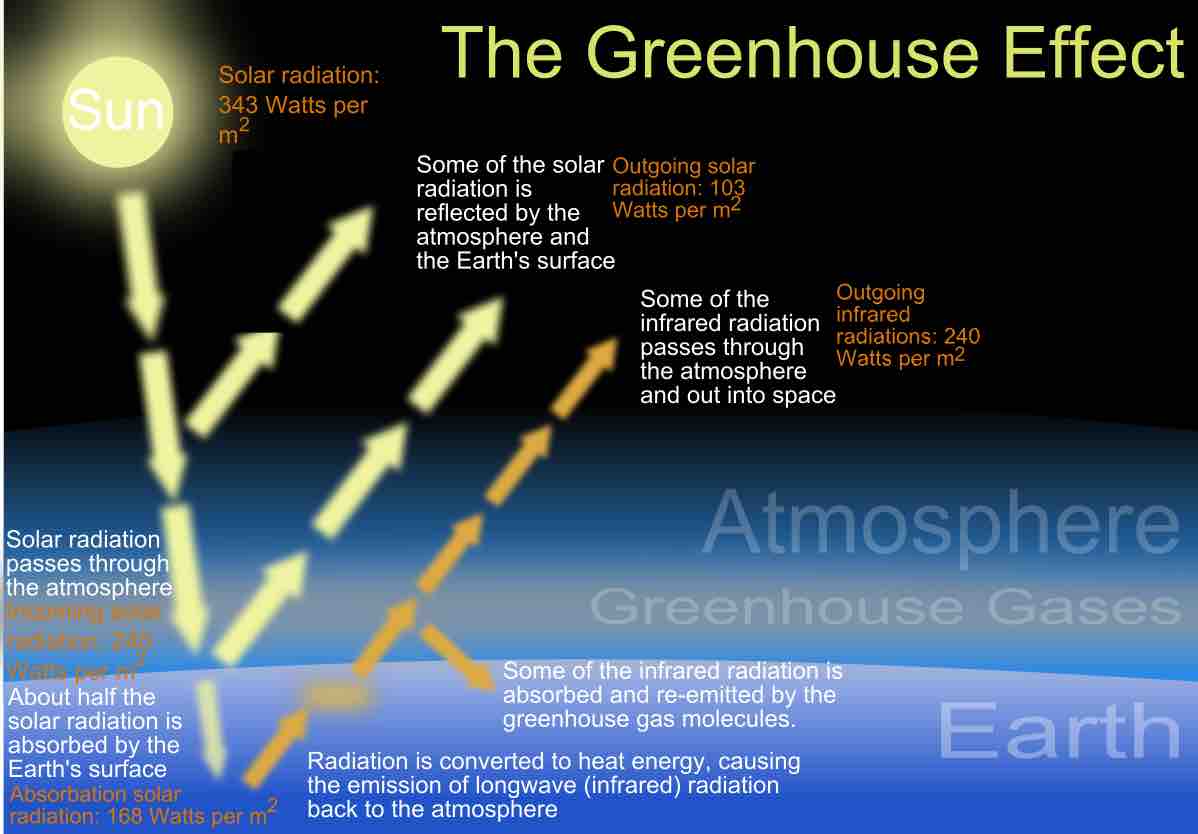
The greenhouse effect is an elevation in surface temperatures due to atmospheric gases absorbing and re-radiating thermal energy.

Photochemical smog forms when primary pollutants react with ultraviolet light to create a variety of toxic and reactive compounds.

Radon gas, the result of radium's radioactive decay, can severely compromise indoor air quality.
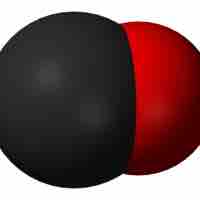
Carbon monoxide and carbon dioxide are products of combustion reactions; in large amounts, carbon monoxide can cause suffocation.
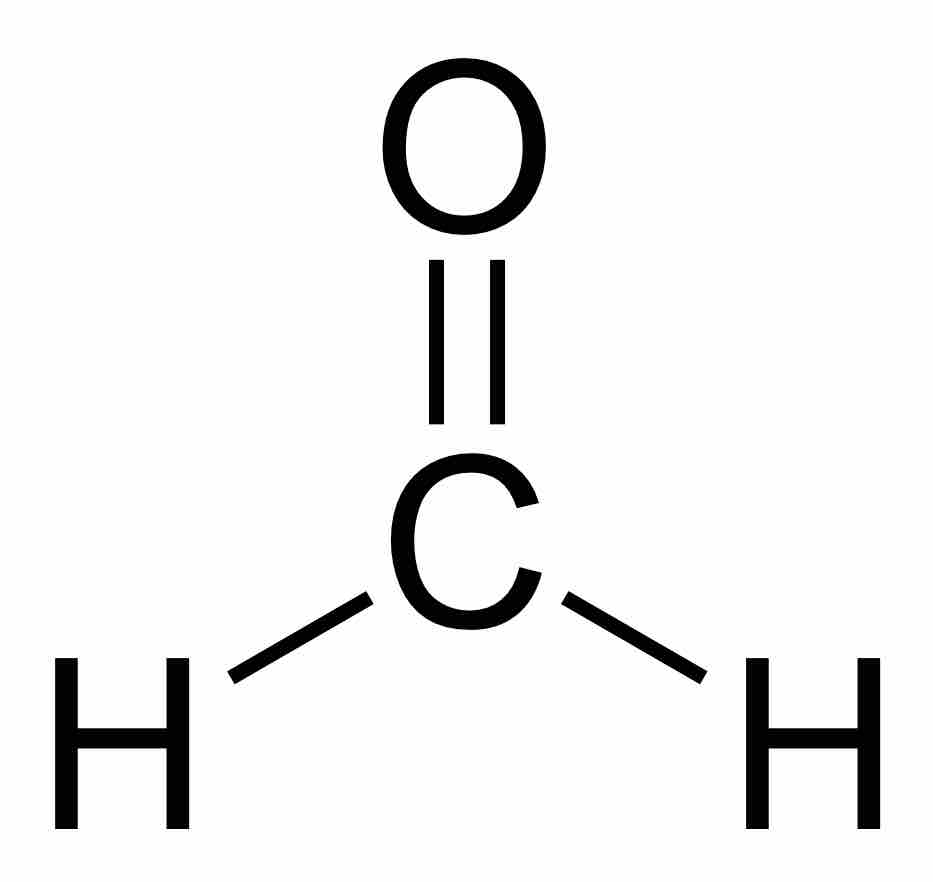
Formaldehyde, the simplest aldehyde, is a common component in building and household materials, and is highly toxic.