The solution of the Schrödinger equation for the hydrogen atom uses the fact that the Coulomb potential produced by the nucleus is isotropic—it is radially symmetric in space and only depends on the distance to the nucleus. Although the resulting energy eigenfunctions (the orbitals) are not necessarily isotropic themselves, their dependence on the angular coordinates follows generally from this isotropy of the underlying potential. The eigenstates of the Hamiltonian (that is, the energy eigenstates) can be chosen as simultaneous eigenstates of the angular momentum operator. This corresponds to the fact that angular momentum is conserved in the orbital motion of the electron around the nucleus. Therefore, the energy eigenstates may be classified by two angular momentum quantum numbers, ℓ and mℓ (both are integers). The angular momentum quantum number ℓ = 0, 1, 2, ... determines the magnitude of the angular momentum. The magnetic quantum number mℓ= −, ..., +ℓ determines the projection of the angular momentum on the (arbitrarily chosen) z-axis and therefore the orientation of the orbital in three-dimensional space.
In addition to mathematical expressions for total angular momentum and angular momentum projection of wavefunctions, an expression for the radial dependence of the wavefunctions must be found. It is only here that the details of the 1/r Coulomb potential enter (leading to Laguerre polynomials in r). This leads to a third quantum number, the principal quantum number n = 1, 2, 3, .... The principal quantum number in hydrogen is related to the atom's total energy. Note that the maximum value of the angular momentum quantum number is limited by the principal quantum number: it can run only up to n − 1, i.e. ℓ = 0, 1, ..., n − 1.
Degeneracy of Different Magnetic Quantum Numbers
Due to angular momentum conservation, states of the same ℓ but different mℓ have the same energy. This holds for all problems with rotational symmetry. For the hydrogen atom, states of the same n but different ℓ are also degenerate (they have the same energy). This is a specific property of hydrogen and is not true for more complicated atoms. These atoms have an effective potential differing from the 1/r form due to the presence of the inner electrons shielding the nucleus potential.
The spin of the electron adds the last quantum number, the projection of the electron's spin angular momentum along the z-axis, which can take on two values. Therefore, any eigenstate of the electron in the hydrogen atom is described fully by four quantum numbers. According to the usual rules of quantum mechanics, the actual state of the electron may be any superposition of these states. This explains also why the choice of z-axis for the directional quantization of the angular momentum vector is immaterial: an orbital of given ℓ and m′ obtained for another preferred axis, z′,can always be represented as a suitable superposition of the various states of different mℓ (but same ℓ) that have been obtained for z.
Using a three-dimensional approach, the following form of the Schrödinger equation can be used to describe the hydrogen atom:
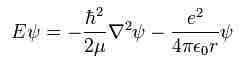
Schroedinger Equation
Three dimensional Schrödinger equation as applied to the H atom.
where
where R are radial functions and theta (
where a0 is the Bohr radius, L are the generalized Laguerre polynomials, and n, l, and m are the principal, azimuthal, and magnetic quantum numbers, respectively.