Overview of Diphtheria
Diphtheria is an upper respiratory tract illness caused by Corynebacterium diphtheriae, a facultative, anaerobic, Gram-positive bacterium. It is characterized by sore throat, low fever, and an adherent membrane (a pseudomembrane) on the tonsils, pharynx, and/or nasal cavity. A milder form of diphtheria can be restricted to the skin . Less common consequences include myocarditis (about 20% of cases) and peripheral neuropathy (about 10% of cases).
Graph of y=x^2
Diphtheria is a contagious disease spread by direct physical contact or breathing the aerosolized secretions of infected individuals. Historically quite common, diphtheria has largely been eradicated in industrialized nations through widespread vaccination. In the United States, for example, there were 52 reported cases of diphtheria between 1980 and 2000; between 2000 and 2007, there were only three cases as the diphtheria–pertussis–tetanus (DPT) vaccine is recommended for all school-age children. Boosters of the vaccine are recommended for adults, since the benefits of the vaccine decrease with age without constant re-exposure; they are particularly recommended for those traveling to areas where the disease has not been eradicated.
Advanced Cases of Diphtheria
In cases that progress beyond a throat infection, diphtheria toxin spreads through the blood and can lead to potentially life-threatening complications that affect other organs, such as the heart and kidneys. The toxin can cause damage to the heart that affects its ability to pump blood or the kidneys' ability to clear wastes. It can also cause nerve damage, eventually leading to paralysis. About 40% to 50% of those left untreated can die.
Diphtheria toxin is produced by C. diphtheriae only when it is infected with a bacteriophage that integrates the toxin-encoding genetic elements into the bacteria. Diphtheria toxin is a single, 60,000 dalton molecular weight protein composed of two peptide chains, fragment A and fragment B, held together by a disulfide bond. Fragment B is a recognition subunit that gains the toxin entry into the host cell by binding to the EGF-like domain of heparin-binding EGF-like growth factor (HB-EGF) on the cell surface. This signals the cell to internalize the toxin within an endosome via receptor-mediated endocytosis. Inside the endosome, the toxin is split by a trypsin-like protease into its individual A and B fragments. The acidity of the endosome causes fragment B to create pores in the endosome membrane, thereby catalyzing the release of fragment A into the cell's cytoplasm.
Fragment A inhibits the synthesis of new proteins in the affected cell. It does this by catalyzing ADP-ribosylation of elongation factor EF-2—a protein that is essential to the translation step of protein synthesis. This ADP-ribosylation involves the transfer of an ADP-ribose from NAD+ to a diphthamide (a modified histidine) residue within the EF-2 protein. Since EF-2 is needed for the moving of tRNA from the A-site to the P-site of the ribosome during protein translation, ADP-ribosylation of EF-2 prevents protein synthesis. ADP-ribosylation of EF-2 is reversed by giving high doses of nicotinamide (a form of vitamin B3), since this is one of the reaction's end-products, thus high amounts will drive the reaction in the opposite direction counteracting the toxin.
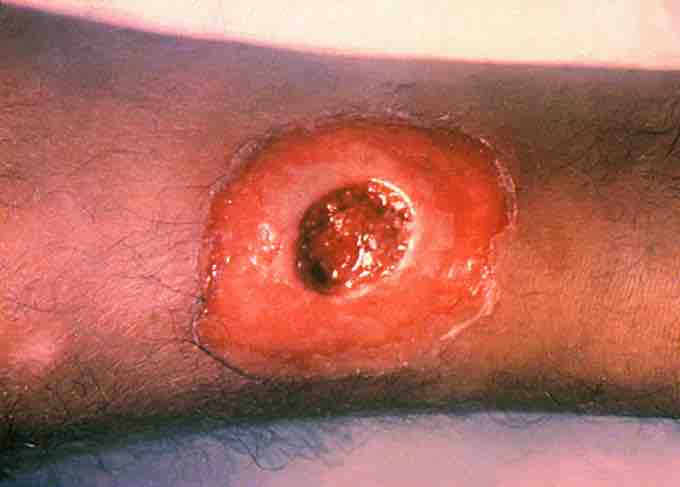
Diphtheria toxin induced lesion
Corynebacterium diphtheriae produces toxins that can affect the skin by causing skin lesions, as shown here.