Posttranslational modification (PTM) is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins. A protein (also called a polypeptide) is a chain of amino acids. During protein synthesis, 20 different amino acids can be incorporated to become a protein. After translation, the posttranslational modification of amino acids extends the range of functions of the protein by attaching it to other biochemical functional groups (such as acetate, phosphate, various lipids, and carbohydrates), changing the chemical nature of an amino acid (e.g., citrullination), or making structural changes (e.g., formation of disulfide bridges).
Also, enzymes may remove amino acids from the amino end of the protein, or cut the peptide chain in the middle. For instance, the peptide hormone insulin is cut twice after disulfide bonds are formed, and a propeptide is removed from the middle of the chain; the resulting protein consists of two polypeptide chains connected by disulfide bonds. Also, most nascent polypeptides start with the amino acid methionine because the "start" codon on mRNA also codes for this amino acid. This amino acid is usually taken off during post-translational modification.
Aside from the 22 standard amino acids, there are many other amino acids that are called non-proteinogenic or non-standard . Those either are not found in proteins (e.g., carnitine, GABA), or are not produced directly and in isolation by standard cellular machinery (e.g., hydroxyproline and selenomethionine).
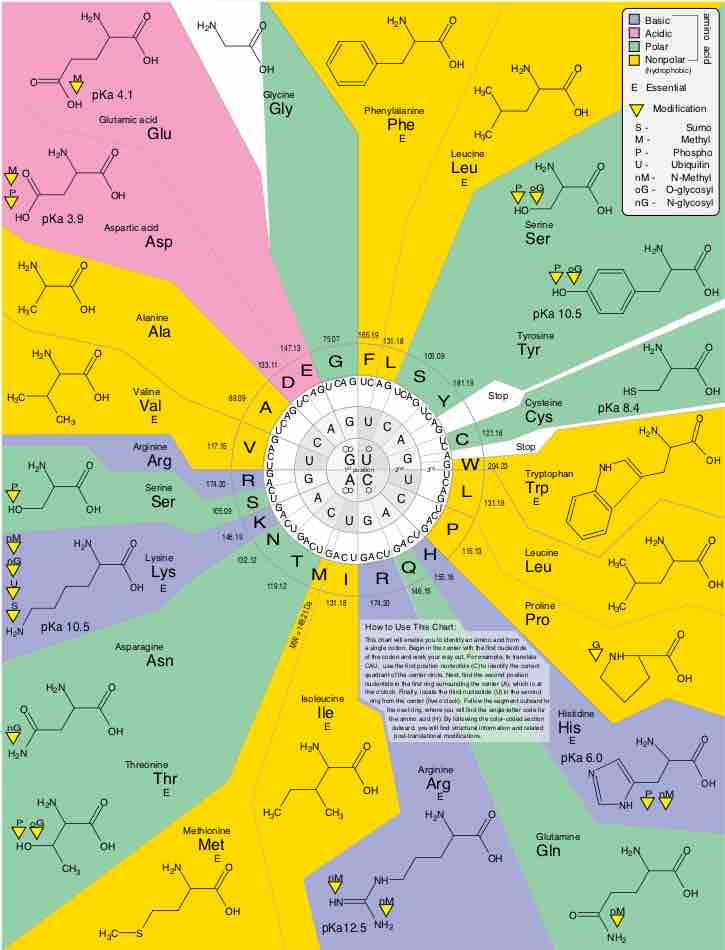
Genetic code
The genetic code diagram showing the amino acid residues as target of modification.
Non-standard amino acids that are found in proteins are formed by post-translational modification, which is modification after translation during protein synthesis. These modifications are often essential for the function or regulation of a protein. For example, the carboxylation of glutamate allows for better binding of calcium cations, and the hydroxylation of proline is critical for maintaining connective tissues.
Another example is the formation of hypusine in the translation initiation factor EIF5A through modification of a lysine residue. Such modifications can also determine the localization of the protein. For instance, the addition of long hydrophobic groups can cause a protein to bind to a phospholipid membrane.
It is important to compare the structures of alanine and beta alanine. In alanine, the side-chain is a methyl group; in beta alanine, the side-chain contains a methylene group connected to an amino group, and the alpha carbon lacks an amino group. The two amino acids, therefore, have the same formulae but different structures.
Some nonstandard amino acids are not found in proteins. Examples include lanthionine, 2-aminoisobutyric acid, dehydroalanine, and the neurotransmitter gamma-aminobutyric acid. Nonstandard amino acids often occur as intermediates in the metabolic pathways for standard amino acids. For example, ornithine and citrulline occur in the urea cycle, which is part of amino acid catabolism. A rare exception to the dominance of α-amino acids in biology is the β-amino acid beta alanine (3-aminopropanoic acid), which is used in plants and microorganisms in the synthesis of pantothenic acid (vitamin B5), a component of coenzyme A.