Concept
Version 8
Created by Boundless
Order to Disorder
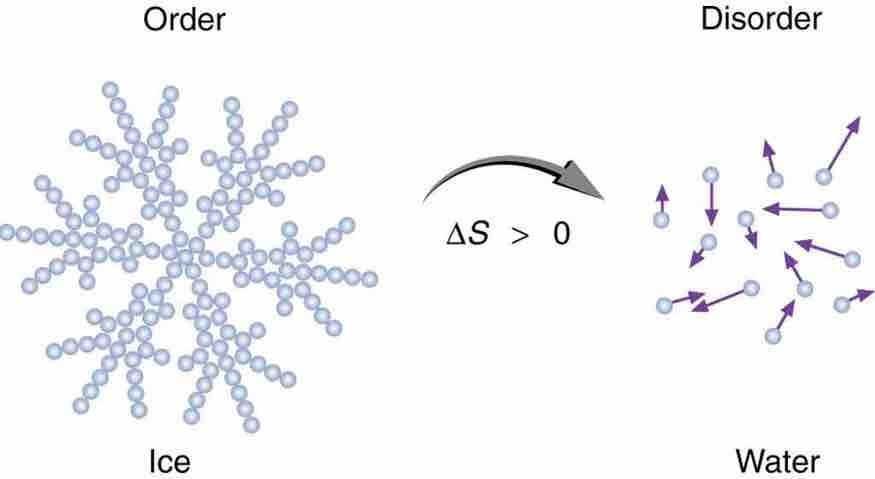
Entropy of Ice
When ice melts, it becomes more disordered and less structured. The systematic arrangement of molecules in a crystal structure is replaced by a more random and less orderly movement of molecules without fixed locations or orientations. Its entropy increases because heat transfer occurs into it. Entropy is a measure of disorder.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, College Physics. February 13, 2013."
http://cnx.org/content/m42237/latest/?collection=col11406/latest
OpenStax CNX
CC BY 3.0.