Molarity
In chemistry, molar concentration, or molarity, is defined as moles of solute per total liters of solution. This is an important distinction; the volume in the definition of molarity refers to the volume of the solution, and not the volume of the solvent. The reason for this is because one liter of solution usually contains either slightly more or slightly less than 1 liter of solvent, due to the presence of the solute. The SI unit for molarity is is mol/m3; however, you will almost always encounter molarity with the units of mol/L. A solution of concentration 1 mol/L is also denoted as "1 molar" (1 M). Mol/L can also be written in the following ways (however, mol/L, or simply M, is most common):
1 mol/L = 1 M = 1 mol/dm3 = 1 mol dm−3 = 1000 mol/m3
It is important to distinguish moles from molarity; molarity is a measurement of concentration while moles are a measure of the amount of substance present at a given time.
Molarity
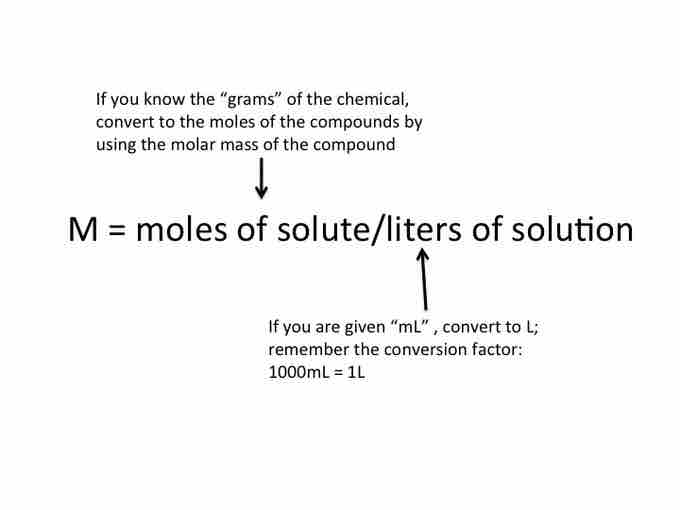
Molarity is a measurement of concentration, with units of mol solute per liter solution.
Using Molarity in Calculations Involving Solutions
Molarity can be used in a various calculations involving solutions. The following formula is very useful, as it relates the molarity of the solution, the total volume of the solution (in liters), and the number of moles solute:
Example 1
A student pipettes a 100 mL sample of a 1.5 M solution of potassium bromide. How many moles of potassium bromide are contained in the sample?
(1.5 M)(0.100 L) = mol
mol = 0.15 mol KBr
Notice in the example above that volume must be converted to L from mL.
You might notice that the above formula bears some resemblance to our dilution formula:
Because we now know that MV = mol, we can simplify our the dilution formula to the following:
This shouldn't surprise us. After all, in any dilution, what changes is the amount of solvent, while the number of moles of solute remains constant throughout.
Example 2
What is the molarity of a solution containing 0.32 moles of NaCl in 3.4 liters of solution?