Concept
Version 8
Created by Boundless
Supercritical Fluids
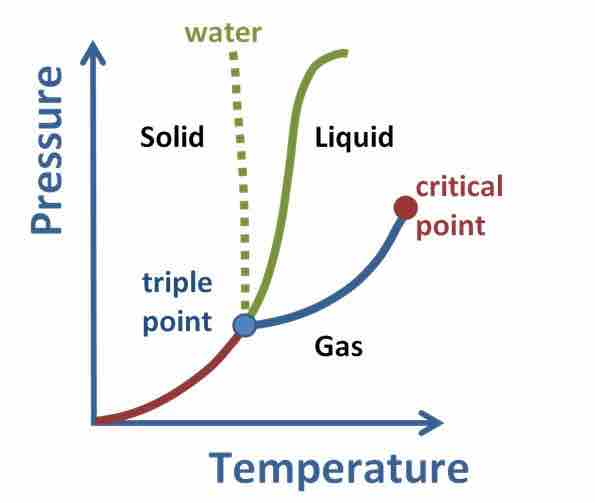
Phase Diagram for a Substance
The figure highlights the critical point, above which (in either temperature or pressure) the substance does not exist in either the liquid or gas phase. Under those conditions it is called a "supercritical fluid," and has properties between those of a liquid and a gas.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Phase Diagram For Pure Substance."
http://commons.wikimedia.org/wiki/File:Phase_diagram_for_pure_substance.JPG
Wikimedia Commons
CC BY-SA 3.0.