Concept
Version 11
Created by Boundless
Properties of Aromatic Compounds
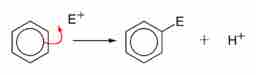
Electrophilic Aromatic Substitution
The electron-rich benzene makes a bond with an electron-deficient chemical species (E+, the electrophile) which takes the place of an H-atom in the original structure. The reaction preserves the pi system of electrons and therefore the aromatic character of the benzene ring.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Electrophilic-aromatic-substitution-general."
http://commons.wikimedia.org/wiki/File:Electrophilic-aromatic-substitution-general.png
Wikimedia Commons
Public domain.