Structure of the Periodic Table
The periodic table displays all the chemical elements organized by atomic number and electron configuration. Specifically, elements are presented by increasing atomic number. The main body of the table is an 18 by 7 grid, and elements with the same number of valence electrons are kept together in groups (columns), such as the halogens and the noble gases. The table has four distinct rectangular areas, or blocks. The f block is usually included below the main table rather than within it to minimize the table's width. The trends in the periodic table can help predict the properties of various elements and the relations between properties. As a result, the periodic table is a useful framework for analyzing chemical behavior and as such is widely used in chemistry and other sciences.
Groups
A group, or family, is a vertical column in the periodic table. Elements in the same group show patterns in atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase: since there are more filled energy levels, valence electrons are found farther from the nucleus. From top to bottom, each successive element has a lower ionization energy because it is easier to remove an electron since the atoms are less tightly bound. Similarly, from top to bottom, elements decrease in electronegativity due to an increasing distance between valence electrons and the nucleus. There are exceptions to these trends however – in Group 11, for example, the electronegativity increases down the group.
Periods
Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity. Moving left to right across a period, from the alkali metals to the noble gases, atomic radius usually decreases. This is because each successive element has an additional proton and electron, which causes the electrons to be drawn closer to the nucleus. The additional proton increases the effective nuclear charge to a greater extent than the addition of an extra electron to an already partially-filled shell. The decrease in atomic radius also causes ionization energy to increase from left to right across a period: the more tightly bound an element is, the more energy is required to remove an electron. Electronegativity increases in the same manner as ionization energy because of the pull exerted on the electrons by the nucleus. Electron affinity also shows a slight trend across a period: metals (the left side of a period) generally have a lower electron affinity than nonmetals (the right side of a period), with the exception of the noble gases which have an electron affinity of zero.
Blocks
Because of the importance of the outermost electron shell, the different regions of the periodic table are sometimes referred to as blocks, named according to the subshell in which the "last" electron resides. The s block includes the first two groups (alkali metals and alkaline earth metals) as well as hydrogen and helium. The p block includes the last six groups, which are Groups 13 to 18 in IUPAC (3A to 8A in American), and contains, among others, all of the metalloids. The d block includes Groups 3 to 12 in IUPAC (or 3B to 2B in American group numbering) and contains all of the transition metals. The f block, usually offset below the rest of the periodic table, includes the lanthanides and actinides.
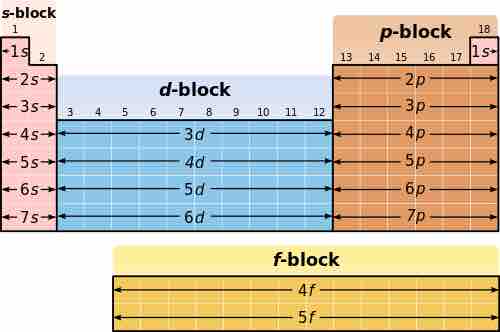
Blocking in the periodic table
The periodic table can be broken into blocks, corresponding to the highest energy electrons.
Periodicity
The primary determinant of an element's chemical properties is its electron configuration, particularly that of the valence shell electrons. For instance, all atoms with four valence electrons occupying p orbitals will share some similar characteristics. The type of orbital in which the atom's outermost electrons reside determines the "block" to which that element belongs. The number of valence shell electrons determines the family, or group, to which the element belongs. The total number of electron shells an atom has determines the period to which it belongs. Each shell is divided into different subshells. Since the outermost electrons determine chemical properties, those with the same number of valence electrons are generally grouped together.