Benzoate catabolism is a series of chemical reactions resulting in the breakdown of benzoate. The purple non-sulphur (PNS) bacteria Rhodobacter sphaeroides is able to produce hydrogen from a wide range of organic compounds (chiefly organic acids) and light. The photo-system required for hydrogen production in Rhodobacter (PS-I) differ from its oxygenic photosystem (PS-II) due to the requirement of organic acids and the inability to oxidize water. In the absence of water-splitting, photosynthesis is anoxygenic. Therefore, hydrogen production is sustained without inhibition from generated oxygen. In PNS bacteria, hydrogen production is due to catalysis by nitrogenase. Hydrogenases are also present but the production of hydrogen by [FeFe]-hydrogenase is less than ten times the hydrogen uptake by [NiFe]-hydrogenase. Only under nitrogen-deficient conditions is nitrogenase activity sufficient to overcome uptake hydrogenase activity, resulting in net generation of hydrogen.
Rhodococcus is a genus of aerobic, nonsporulating, nonmotile Gram-positive bacteria closely related to Mycobacteria and Corynebacteria . While a few species are pathogenic, most are benign and have been found to thrive in a broad range of environments, including soil, water, and eukaryotic cells. Fully sequenced in October 2006, the genome is known to be 9.7 megabasepairs long and 67% G/C. Strains of Rhodococcus are applicably important owing to their ability to catabolize a wide range of compounds and produce bioactive steroids, acrylamide, and acrylic acid, and their involvement in fossil fuel biodesulfurization. This genetic and catabolic diversity is not only due to the large bacterial chromosome, but also to the presence of three large linear plasmids. Rhodococcus is also an experimentally advantageous system owing to a relatively fast growth rate and simple developmental cycle. However, as it stands now, Rhodococcus is not well characterized. Another important application of Rhodococcus comes from bioconversion, using biological systems to convert cheap starting material into more valuable compounds. This use of Rhodococcus is borne out of its ability to metabolize harmful environmental pollutants, such as toluene, naphthalene, herbicides, and PCBs. Rhodococci typically metabolize aromatic substrates by first oxygenating the aromatic ring to form a diol (two alcohol groups). Then, the ring is cleaved with intra/extradiol mechanisms, opening the ring and exposing the substrate to further metabolism. Since the chemistry here is very stereospecific, the diols are created with predictable chirality. While controlling the chirality of chemical reaction presents a significant challenge for synthetic chemists, biological processes can be used instead to faithfully produce chiral molecules in cases where direct chemical synthesis is infeasible or inefficient. An example of this is the use of Rhodococcus to produce indene, a precursor to the AIDS drug CrixivanTM, a protease inhibitor, and containing two of the five chiral centers needed in the complex.
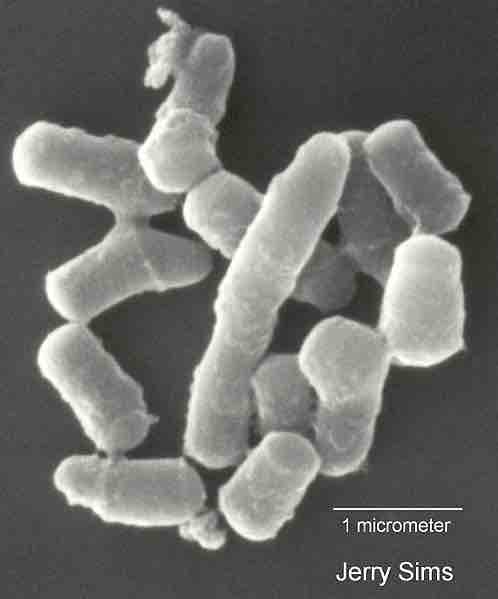
Scanning electron micrograph of Rhodococcus sp. strain Q1
Rhodococcus sp. strain Q1 grown on quinoline - the organism can use quinoline as a sole source of carbon, nitrogen and energy, tolerating concentrations up to 3.88 millimoles per liter.