A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes. Cofactors can be considered "helper molecules" that assist in biochemical transformations .
Cofactor
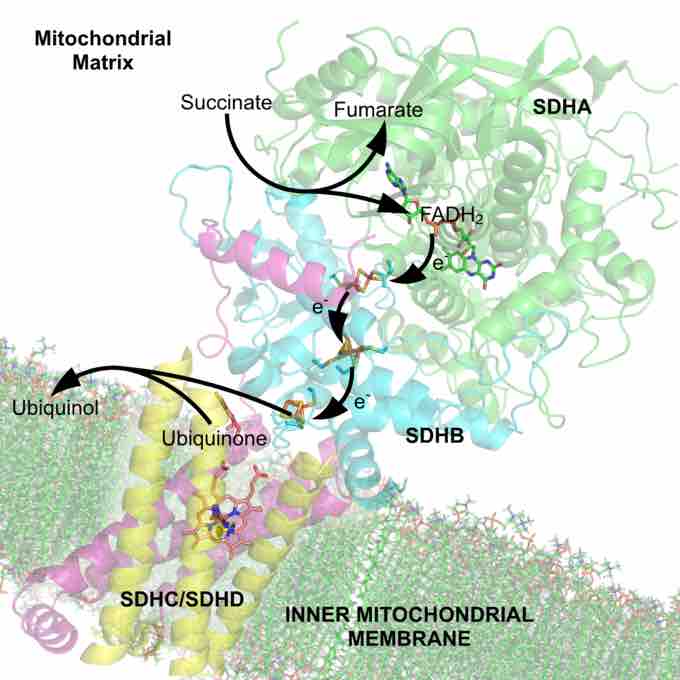
The succinate dehydrogenase complex showing several cofactors, including flavin, iron-sulfur centers, and heme.
Cofactors are either organic or inorganic. They can also be classified depending on how tightly they bind to an enzyme, with loosely-bound cofactors termed coenzymes and tightly-bound cofactors termed prosthetic groups. Some sources also limit the use of the term "cofactor" to inorganic substances. An inactive enzyme without the cofactor is called an apoenzyme, while the complete enzyme with cofactor is the holoenzyme.
Some enzymes or enzyme complexes require several cofactors. For example, the multienzyme complex pyruvate dehydrogenase at the junction of glycolysis and the citric acid cycle requires five organic cofactors and one metal ion: loosely bound thiamine pyrophosphate (TPP), covalently bound lipoamide and flavin adenine dinucleotide (FAD), and the cosubstrates nicotinamide adenine dinucleotide (NAD+) and coenzyme A (CoA), and a metal ion (Mg2+).
Organic cofactors are often vitamins or are made from vitamins. Many contain the nucleotide adenosine monophosphate (AMP) as part of their structures, such as ATP, coenzyme A, FAD, and NAD+. This common structure may reflect a common evolutionary origin as part of ribozymes in an ancient RNA world. It has been suggested that the AMP part of the molecule can be considered a kind of "handle" by which the enzyme can "grasp" the coenzyme to switch it between different catalytic centers.
Cofactors can be divided into two broad groups: organic cofactors, such as flavin or heme, and inorganic cofactors, such as the metal ions Mg2+, Cu+, Mn2+, or iron-sulfur clusters.
Vitamins can serve as precursors to many organic cofactors (e.g., vitamins B1, B2, B6, B12, niacin, folic acid) or as coenzymes themselves (e.g., vitamin C). However, vitamins do have other functions in the body. Many organic cofactors also contain a nucleotide, such as the electron carriers NAD and FAD, and coenzyme A, which carries acyl groups. Most of these cofactors are found in a huge variety of species, and some are universal to all forms of life. An exception to this wide distribution is a group of unique cofactors that evolved in methanogens, which are restricted to this group of archaea.
Metabolism involves a vast array of chemical reactions, but most fall under a few basic types of reactions that involve the transfer of functional groups. This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions. These group-transfer intermediates are the loosely-bound organic cofactors, often called coenzymes.
Each class of group-transfer reaction is carried out by a particular cofactor, which is the substrate for a set of enzymes that produce it and a set of enzymes that consume it. An example of this is the dehydrogenases that use nicotinamide adenine dinucleotide (NAD+) as a cofactor. Here, hundreds of separate types of enzymes remove electrons from their substrates and reduce NAD+ to NADH. This reduced cofactor is then a substrate for any of the reductases in the cell that require electrons to reduce their substrates.
Therefore, these cofactors are continuously recycled as part of metabolism. As an example, the total quantity of ATP in the human body is about 0.1 mole. This ATP is constantly being broken down into ADP, and then converted back into ATP. Therefore, at any given time, the total amount of ATP + ADP remains fairly constant. The energy used by human cells requires the hydrolysis of 100 to 150 moles of ATP daily, which is around 50 to 75 kg. In typical situations, humans use up their body weight of ATP over the course of the day. This means that each ATP molecule is recycled 1,000 to 1,500 times daily.
The term is used in other areas of biology to refer more broadly to non-protein (or even protein) molecules that either activate, inhibit, or are required for the protein to function. For example, ligands such as hormones that bind to and activate receptor proteins are termed cofactors or coactivators, whereas molecules that inhibit receptor proteins are termed corepressors.