Cellular Respiration
Cellular respiration is a set of metabolic reactions and processes that take place within the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP). The reactions involved in this respiration are considered to be catabolic reactions that release energy as larger molecules are broken down into smaller ones and high-energy bonds are broken. Respiration is one of the key ways a cell gains useful energy to fuel cellular activity .
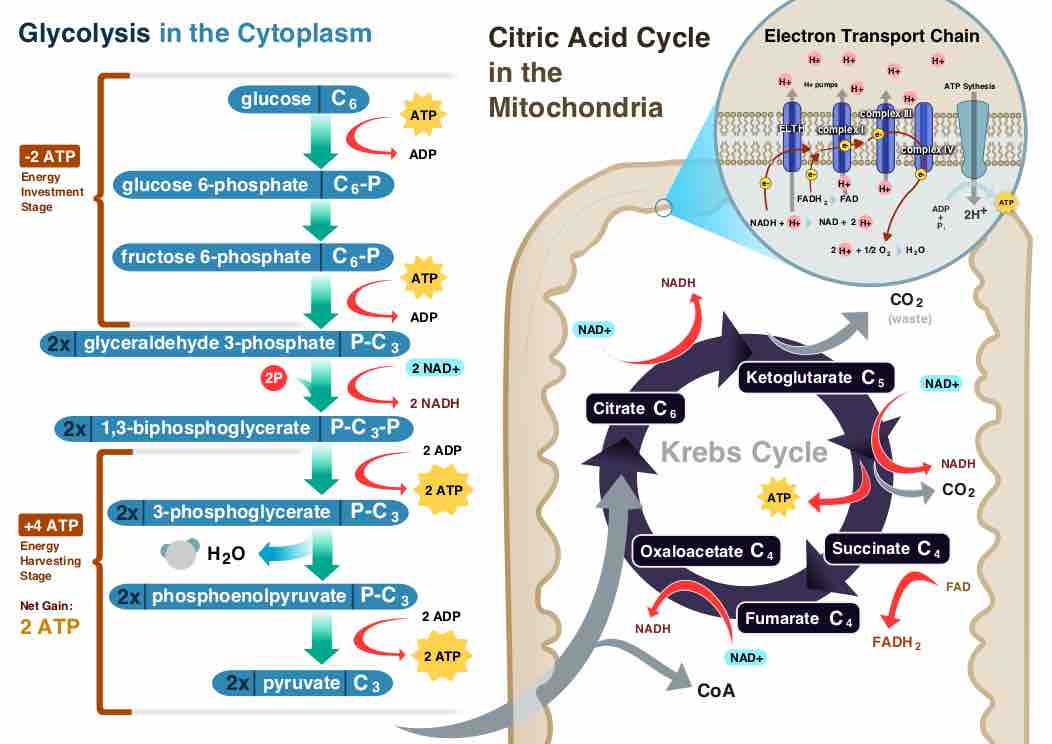
Overview of Cellular Respiration
A diagram of cellular respiration including glycolysis, Krebs cycle (AKA citric acid cycle), and the electron transport chain.
Chemically, cellular respiration is considered an exothermic redox reaction. The overall reaction is broken into many smaller ones when it occurs in the body. Most of these smaller reactions are redox reactions themselves. Although technically, cellular respiration is a combustion reaction, it does not resemble one when it occurs in a living cell. This is because it occurs in many separate steps. While the overall reaction is a combustion reaction, no single reaction that comprises it is a combustion reaction.
Aerobic and Anaerobic Reactions
Aerobic reactions require oxygen for ATP generation. Although carbohydrates, fats and proteins can be used as reactants, the preferred method is the process of glycolysis. During glycolysis, pyruvate is formed from glucose metabolism. During aerobic conditions, the pyruvate enters the mitochondrion to be fully oxidized by the Krebs cycle. The products of the Krebs cycle include energy in the form of ATP (via substrate level phosphorylation), NADH, and FADH2.
The simplified reaction is as follows:
C6H12O6 (s) + 6 O2 (g) → 6 CO2 (g) + 6 H2O (l) + heat
ΔG = -2880 kJ per mole of C6H12O6
A negative ΔG indicates that the reaction can occur spontaneously.
Aerobic metabolism is up to 15 times more efficient than anaerobic metabolism, which yields two molecules ATP per one molecule glucose. Both types of metabolism share the initial pathway of glycolysis, but aerobic metabolism continues with the Krebs cycle and oxidative phosphorylation. In eukaryotic cells, the post-glycolytic reactions take place in the mitochondria, while in prokaryotic cells, these reactions take place in the cytoplasm .
Humans use of prokaryotes
This is a microscopic image of Bacillus subtilis (ATCC 6633) with a gram staining of magnification: 1,000. The oval, unstained structures are spores.
Glycolysis
Glycolysis takes place in the cytosol, does not require oxygen, and can therefore function under anaerobic conditions. The process converts one molecule of glucose into two molecules of pyruvate, generating energy in the form of two net molecules of ATP. Four molecules of ATP per glucose are actually produced, but two of these are consumed as part of the preparatory phase. The initial phosphorylation of glucose is required to destabilize the molecule for cleavage into two pyruvate. During the pay-off phase of glycolysis, four phosphate groups are transferred to ADP by substrate-level phosphorylation to make four ATP, and two NADH are produced when the pyruvate are oxidized. The overall reaction can be expressed this way:
Glucose + 2 NAD+ + 2 Pi + 2 ADP → 2 pyruvate + 2 NADH + 2 ATP + 2 H+ + 2 H2O + heat
Starting with glucose, one ATP is used to donate a phosphate to glucose to produce glucose 6-phosphate. With the help of glycogen phosphorylase, glycogen can change into glucose 6-phosphate as well. During energy metabolism, glucose 6-phosphate turns into fructose 6-phosphate. With the help of phosphofructokinase, an additional ATP can be used to turn phosphorylate fructose 6-phosphate into fructose 1, 6-diphosphate. Fructose 1, 6-diphosphate then splits into two phosphorylated molecules with three carbon chains that later degrades into pyruvate.
Making Proton Gradients
Some archaea, the most notable ones being halobacteria, make proton gradients by pumping in protons from the environment. They are able to do this with the help of the solar-driven enzyme bacteriorhodopsin, which is used to drive the molecular motor enzyme ATP synthase to make the necessary conformational changes required to synthesize ATP. By running ATP synthase in reverse, proton gradients are also made by bacteria and are used to drive flagella. The F1FO ATP synthase is a reversible enzyme. Large enough quantities of ATP cause it to create a transmembrane proton gradient. This is used by fermenting bacteria, which lack an electron transport chain, and which hydrolyze ATP to make a proton gradient. Bacteria use these gradients for flagella and for the transportation of nutrients into the cell. In respiring bacteria under physiological conditions, ATP synthase, in general, runs in the opposite direction. This creates ATP while using the proton motive force created by the electron transport chain as a source of energy. The overall process of creating energy in this fashion is termed oxidative phosphorylation.