A biofilm is an aggregate of microorganisms in which cells adhere to each other on a surface. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS).
Microbes form a biofilm in response to many factors, which may include cellular recognition of specific or non-specific attachment sites on a surface, nutritional cues, or in some cases, by exposure of planktonic cells to sub-inhibitory concentrations of antibiotics. When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large suites of genes are differentially regulated .
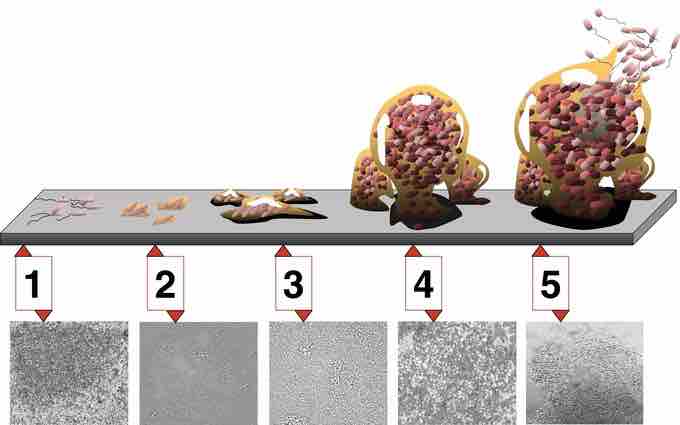
Biofilm development
5 stages of biofilm development. Stage 1, initial attachment; stage 2, irreversible attachment; stage 3, maturation I; stage 4, maturation II; stage 5, dispersion. Each stage of development in the diagram is paired with a photomicrograph of a developing Pseudomonas aeruginosa biofilm. All photomicrographs are shown to same scale.
Biofilms are ubiquitous. Nearly every species of microorganism, not only bacteria and archaea, have mechanisms by which they can adhere to surfaces and to each other. Biofilms will form on virtually every non-shedding surface in a non-sterile aqueous (or very humid) environment.
Biofilms have been found to be involved in a wide variety of microbial infections in the body, by one estimate in 80% of all infections. Infectious processes in which biofilms have been implicated include common problems such as urinary tract infections, catheter infections , middle-ear infections, formation of dental plaque, gingivitis, and coating contact lenses. Biofilms have also been implicated in less common but more lethal processes such as endocarditis, infections in cystic fibrosis, and infections of permanent indwelling devices such as joint prostheses and heart valves.
Staphylococcus aureus biofilm
Staphylococcus aureus forming a biofilm on a catheter.
More recently it has been noted that bacterial biofilms may impair cutaneous wound healing and reduce topical antibacterial efficiency in healing or treating infected skin wounds. It has recently been shown that biofilms are present on the removed tissue of 80% of patients undergoing surgery for chronic sinusitis. The patients with biofilms were shown to have been denuded of cilia and goblet cells, unlike the controls without biofilms who had normal cilia and goblet cell morphology. Biofilms were also found on samples from two of 10 healthy controls mentioned. The species of bacteria from interoperative cultures did not correspond to the bacteria species in the biofilm on the respective patient's tissue. In other words, the cultures were negative though the bacteria were present.
Biofilms can also be formed on the inert surfaces of implanted devices such as catheters, prosthetic cardiac valves, and intrauterine devices. New staining techniques are being developed to differentiate bacterial cells growing in living animals, e.g. from tissues with allergy-inflammations.
Pseudomonas aeruginosa biofilms
The achievements of medical care in industrialized societies are markedly impaired due to chronic opportunistic infections that have become increasingly apparent in immunocompromised patients and the aging population. Chronic infections remain a major challenge for the medical profession and are of great economic relevance because traditional antibiotic therapy is usually not sufficient to eradicate these infections.
Pseudomonas aeruginosa is not only an important opportunistic pathogen and causative agent of emerging nosocomial infections but can also be considered a model organism for the study of diverse bacterial mechanisms that contribute to bacterial persistence. In this context the elucidation of the molecular mechanisms responsible for the switch from planktonic growth to a biofilm phenotype and the role of inter-bacterial communication in persistent disease should provide new insights. It should help researchers learn about the pathogenicity of P. aeruginosa, contribute to a better clinical management of chronically infected patients, and lead to the identification of new drug targets for the development of alternative anti-infective treatment strategies.
Dental plaque
Dental plaque is a biofilm that adheres to teeth surfaces and consists of bacterial cells, salivary polymers, and bacterial extracellular products. This accumulation of microorganisms subject the teeth and gingival tissues to high concentrations of bacterial metabolites which results in dental disease. The biofilms attached to the surfaces of some dental alloys, impression materials, dental implants, restorative and cement materials play an essential role concerning the biofilms establishment dynamics toward the physical-chemical properties of the materials which biofilms are attached to.
Legionellosis
Legionella bacteria are known to grow under certain conditions in biofilms, in which they are protected against disinfectants. Workers in cooling towers, persons working in air conditioned rooms, and people taking a shower are exposed to Legionella by inhalation when the systems are not well designed, constructed, or maintained. Neisseria gonorrhoeae is an exclusive human pathogen. Recent studies have demonstrated that it utilizes two distinct mechanisms for entry into human urethral and cervical epithelial cells involving different bacterial surface ligands and host receptors. In addition, it has been demonstrated that the gonococcus can form biofilms on glass surfaces and over human cells. There is evidence for the formation of gonococcal biofilms on human cervical epithelial cells during natural disease. Evidence also suggests that the outer membrane blebbing by the gonococcus is crucial in biofilm formation over human cervical epithelial cells.