The Rutherford model is a model of the atom named after Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested, according to Rutherford's 1911 analysis, that J. J. Thomson's so-called "plum pudding model" of the atom was incorrect. Rutherford's new model for the atom, based on the experimental results, contained the new features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom. This central volume also contained the bulk of the atom's mass . This region would later be named the "nucleus. "
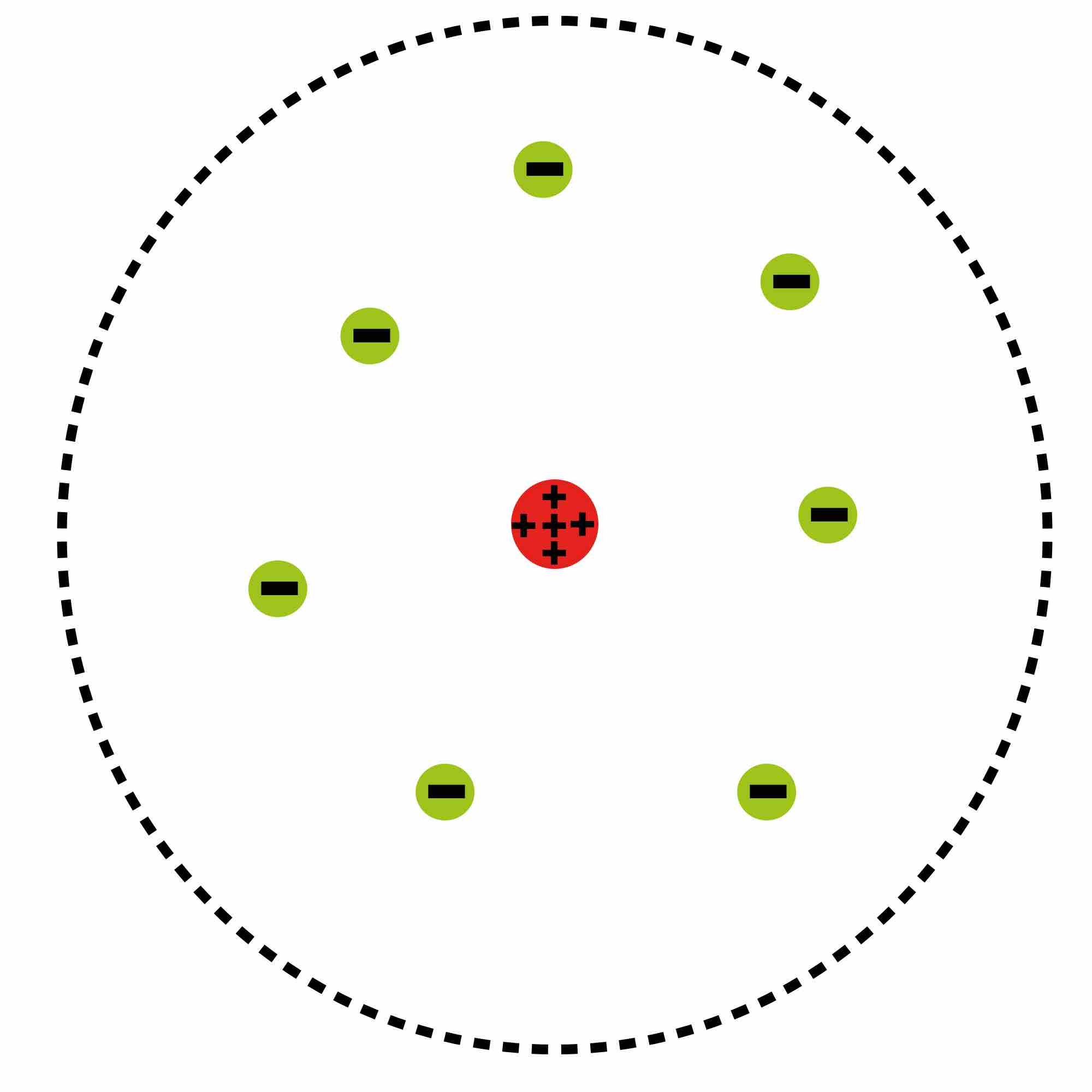
Atomic Planetary Model
Basic diagram of the atomic planetary model; electrons are in green, and the nucleus is in red
In 1911, Rutherford designed an experiment to further explore atomic structure using the alpha particles emitted by a radioactive element . Following his direction, Geiger and Marsden shot alpha particles with large kinetic energies toward a thin foil of gold. Measuring the pattern of scattered particles was expected to provide information about the distribution of charge within the atom. Under the prevailing plum pudding model, the alpha particles should all have been deflected by, at most, a few degrees. However, the actual results surprised Rutherford. Although many of the alpha particles did pass through as expected, many others were deflected at small angles while others were reflected back to the alpha source.

Thomson's Model vs. Rutherford's Model
Top: Expected results -- alpha particles pass through the plum pudding model of the atom undisturbed. Bottom: Observed results -- a small portion of the particles were deflected, indicating a small, concentrated positive charge. (Note that the image is not to scale; in reality the nucleus is vastly smaller than the electron shell. )
From purely energetic considerations of how far particles of known speed would be able to penetrate toward a central charge of 100 e, Rutherford was able to calculate that the radius of his gold central charge would need to be less than