J. J. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In Thomson's model, the atom is composed of electrons (which Thomson still called "corpuscles," though G. J. Stoney had proposed that atoms of electricity be called electrons in 1894) surrounded by a soup of positive charge to balance the electrons' negative charges, like negatively charged "plums" surrounded by positively charged "pudding" . The electrons (as we know them today) were thought to be positioned throughout the atom in rotating rings. In this model the atom was also sometimes described to have a "cloud" of positive charge.
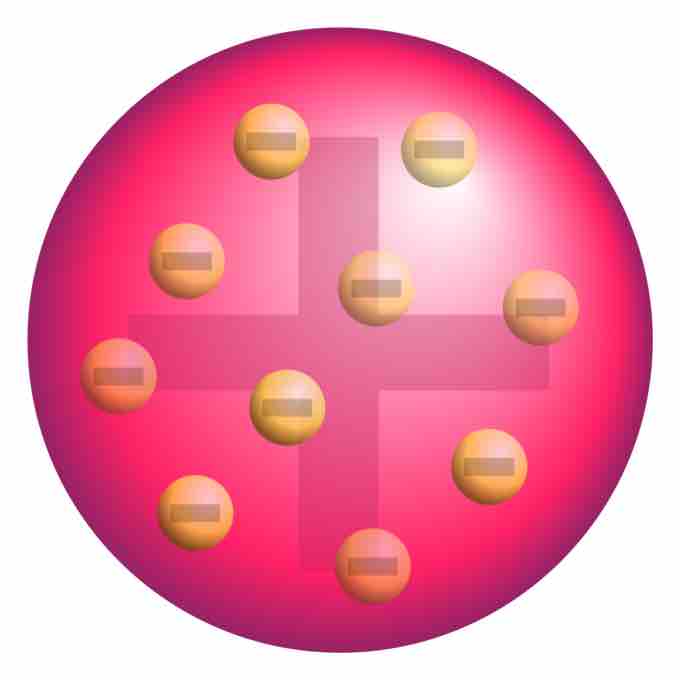
Plum pudding model of the atom
A schematic presentation of the plum pudding model of the atom; in Thomson's mathematical model the "corpuscles" (in modern language, electrons) were arranged non-randomly, in rotating rings.
With this model, Thomson abandoned his earlier "nebular atom" hypothesis, in which the atom was composed of immaterial vortices. Now, at least part of the atom was to be composed of Thomson's particulate negative corpuscles, although the rest of the positively charged part of the atom remained somewhat nebulous and ill-defined.
The 1904 Thomson model was disproved by the 1909 gold foil experiment performed by Hans Geiger and Ernest Marsden. This gold foil experiment was interpreted by Ernest Rutherford in 1911 to suggest that there is a very small nucleus of the atom that contains a very high positive charge (in the case of gold, enough to balance the collective negative charge of about 100 electrons). His conclusions led him to propose the Rutherford model of the atom.