X-Rays
X-rays are electromagnetic waves with wavelengths in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz (3×1016 Hz to 3×1019 Hz) and energies in the range 100 eV to 100 keV . They are shorter in wavelength than UV rays and longer than gamma rays. In many languages, X-radiation is called Röntgen radiation, after Wilhelm Röntgen, who is usually credited as its discoverer, and who had named it X-radiation to signify an unknown type of radiation.
Electromagnetic Spectrum
The electromagnetic spectrum, showing the major categories of electromagnetic waves. The range of frequencies and wavelengths is remarkable. The dividing line between some categories is distinct, whereas other categories overlap. Microwaves encompass the high frequency portion of the radio section of the EM spectrum.
Properties and Applications
X-ray photons carry enough energy to ionize atoms and disrupt molecular bonds. This makes it a type of ionizing radiation and thereby harmful to living tissue. A very high radiation dose over a short amount of time causes radiation sickness, while lower doses can give an increased risk of radiation-induced cancer. In medical imaging this increased cancer risk is generally greatly outweighed by the benefits of the examination. The ionizing capability of X-rays can be utilized in cancer treatment to kill malignant cells using radiation therapy. It is also used for material characterization using X-ray spectroscopy .
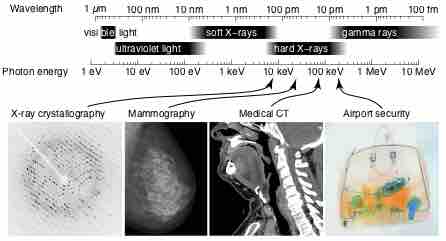
X-Ray Spectrum and Applications
X-rays are part of the electromagnetic spectrum, with wavelengths shorter than those of visible light. Different applications use different parts of the X-ray spectrum.
X-rays with photon energies above 5 to 10 keV (below 0.2-0.1 nm wavelength), are called hard X-rays, while those with lower energy are called soft X-rays. Due to their penetrating ability, hard X-rays are widely used to image the inside of objects (e.g., in medical radiography and airport security). As a result, the term X-ray is metonymically used to refer to a radiographic image produced using this method, in addition to the method itself. Since the wavelength of hard X-rays are similar to the size of atoms, they are also useful for determining crystal structures by X-ray crystallography. By contrast, soft X-rays are easily absorbed in air and the attenuation length of 600 eV (~2 nm) X-rays in water is less than 1 micrometer.
In medical diagnostic applications, the low energy (soft) X-rays are unwanted, since they are totally absorbed by the body, increasing the radiation dose without contributing to the image. Hence, a thin metal sheet, often of aluminum, called an X-ray filter, is usually placed over the window of the X-ray tube, absorbing the low energy part in the spectrum. This is called hardening the beam since it shifts the center of the spectrum towards higher energy (or harder) X-rays.
Distinction Between X-Rays and Gamma Rays
The distinction between X-rays and gamma rays is somewhat arbitrary. The most frequent method of distinguishing between X- and gamma radiation is the basis of wavelength, with radiation shorter than some arbitrary wavelength, such as 10−11 m, defined as gamma rays. The electromagnetic radiation emitted by X-ray tubes generally has a longer wavelength than the radiation emitted by radioactive nuclei. Historically, therefore, an alternative means of distinguishing between the two types of radiation has been by their origin: X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus. There is overlap between the wavelength bands of photons emitted by electrons outside the nucleus, and photons emitted by the nucleus. Like all electromagnetic radiation, the properties of X-rays (or gamma rays) depend only on their wavelength and polarization.