
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/cyclosporiasis.
Cyclosporiasis
[Cyclospora cayetanensis]
Laboratory Diagnosis
Currently, the most practical diagnostic method consists of the identification of oocysts in stool specimens by light microscopy. Other methods are also available or under investigation.
Specimen Processing
Specimens should be refrigerated and sent to the diagnostic laboratory as rapidly as possible. If it is not possible to send the specimen to the laboratory promptly, it should be preserved. Ideally, because a range of tests might be desired, each of which has different requirements of the specimen, the latter should be split in portions which should be respectively:
- fixed in 10% formalin (for direct microscopy, concentration procedures, and preparation of stained smears);
- fixed in 2.5% potassium dichromate (for sporulation assays and molecular diagnosis); and
- frozen without fixation (for molecular diagnosis).
Note: Specimens fixed in sodium acetate-acetic acid formalin can be handled in the same manner as specimens fixed in formalin; however, specimens fixed in polyvinyl alcohol (PVA) are of limited value because they are not usable for concentration procedures.
Cyclospora oocysts can be excreted intermittently and in small numbers. Thus:
- a single negative stool specimen does not rule out the diagnosis; three or more specimens at 2- or 3-day intervals may be required
- concentration procedures should be used to maximize recovery of oocysts. The method most familiar to laboratorians is the formalin-ethyl acetate sedimentation technique (centrifuge for 10 minutes at 500 × g). Other methods can also be used (such as the Sheather's flotation procedure).
Microscopic Examination
The sediment can be examined microscopically with different techniques:
- wet mounts (by conventional light microscopy, which can be enhanced by UV fluorescence microscopy or differential interference contrast [DIC, Nomarsky])
- stained smears (using modified acid-fast stain or a modified safranin stain)
Sporulation Assay
Because of the morphologic similarity between freshly passed, unsporulated Cyclospora oocysts and blue-green algae (cyanobacterium-like bodies), it has been advocated that to confirm the diagnosis of cyclosporiasis, unfixed oocysts should be examined over a 2- to 3-week period for evidence of sporulation. This is accomplished by placing an aliquot of fresh stool in 2.5% potassium dichromate (which reduces bacterial overgrowth), and keeping it under observation for sporulation of the oocysts.*
*As the ability of laboratorians to accurately diagnose cyclosporiasis has improved, the need to do sporulation assays has decreased.
Procedure
Mix stool with 2-3 volumes of potassium dichromate (depending on the stool consistency) and agitate specimen occasionally during the incubation period. Leave an air space over the specimen, for example by turning the specimen tube on its side to increase surface area, to promote aeration. At room temperature, oocysts generally sporulate in 5-14 days, although the percentage of oocysts that sporulate can vary. If the number of oocysts is small, the material held in potassium dichromate can be centrifuged and the pellet examined. Prior to examination, the potassium dichromate solution should be diluted (it quenches fluorescence): add to the specimen an equal volume of distilled/deionized water; or remove the dichromate by centrifugation and washing (use distilled/deionized water or various solutions such as PBS, culture medium). Optimal examination of the specimen, using a wet mount, would include epifluorescence (to identify the oocysts) followed by DIC, phase contrast microscopy, or conventional bright-field microscopy (to identify the sporocysts/sporozoites). Sporulated mature oocysts contain two sporocysts, each of which contains two sporozoites. (Because the sporozoites are tightly packed inside the sporocysts, they are difficult to visualize.)
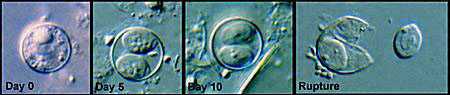
Sporulation of Cyclospora oocysts. The sequence shows, as observed by DIC microscopy of wet mounts: an oocyst passed in fresh stool (Day 0); sporulated oocysts at days 5 (Day 5) and 10 (Day 10), which both contain 2 sporocysts; and a ruptured oocyst (Rupture), with a sporocyst still inside the oocyst and the other sporocyst just outside—the coiled sporozoites are barely visible inside the sporocysts.
Molecular diagnosis
A nested PCR assay targeting the small-subunit ribosomal RNA has been developed.1 Preliminary results indicate that the Cyclospora-specific PCR primers cross react with some Eimeria species and the sensitivity of the assay may be low (62%).2
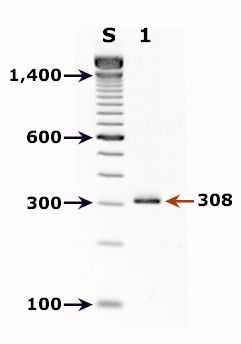
Agarose gel (2%) analysis of a PCR diagnostic test for detection of Cyclospora DNA. PCR was performed using nested primers CYCF1E/CYCR2B (first round) and CYCF3E/CYCR4B (second round).1,2
- Lane S: Molecular base pair standard (100-bp ladder). Black arrows show the size of standard bands.
- Lane 1: Cyclospora positive fecal specimen. The red arrow shows the diagnostic band for Cyclospora cayetanensis (size: 308 bp).
Reference:
Eberhard ML, Pieniazek NJ, and Arrowood MJ. Laboratory diagnosis of Cyclospora infections. Arch Pathol Lab Med 1997;121:792-797.
 ShareCompartir
ShareCompartir
 Key points for laboratory diagnosis of cyclosporiasis -- Cyclospora cayetanensis [PDF, 204 KB, 2 Pages]
Key points for laboratory diagnosis of cyclosporiasis -- Cyclospora cayetanensis [PDF, 204 KB, 2 Pages] Key points for laboratory diagnosis of Cyclospora, Cryptosporidium, and Cystoisospora belli [PDF, 157 KB, 1 Page]
Key points for laboratory diagnosis of Cyclospora, Cryptosporidium, and Cystoisospora belli [PDF, 157 KB, 1 Page]

