Weekly U.S. Influenza Surveillance Report

2017-2018 Influenza Season Week 40 ending October 7, 2017
All data are preliminary and may change as more reports are received.
Background:
The Centers for Disease Control and Prevention’s (CDC) Influenza Division collects, compiles, and analyzes information on influenza activity year-round in the United States and produces FluView, a weekly influenza surveillance report, and FluView Interactive , an application to customize the presentation of influenza surveillance data. The U.S. influenza surveillance system provides information in five categories collected from eight data sources. This is the first report of the 2017-2018 influenza season.
The five categories and eight data components of CDC influenza surveillance are:
- Viral Surveillance: U.S. World Health Organization (WHO) collaborating laboratories, the National Respiratory and Enteric Virus Surveillance System (NREVSS), and human infection with novel influenza A virus case reporting;
- Mortality: National Center for Health Statistics (NCHS) Mortality Surveillance System and influenza-associated pediatric deaths;
- Hospitalizations: Influenza Hospitalization Network (FluSurv-NET) including the Emerging Infections Program (EIP) and three additional states;
- Outpatient Illness Surveillance: U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet);
- Geographic Spread of Influenza: State and territorial epidemiologists’ reports.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
Synopsis:
During week 40 (October 1-7, 2017), influenza activity was low in the United States.
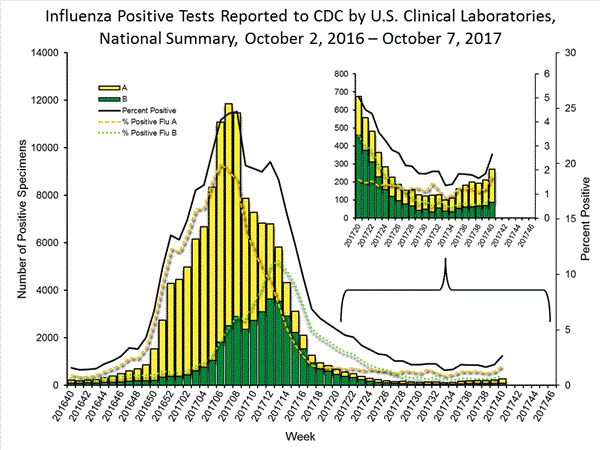
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 40 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories is low.
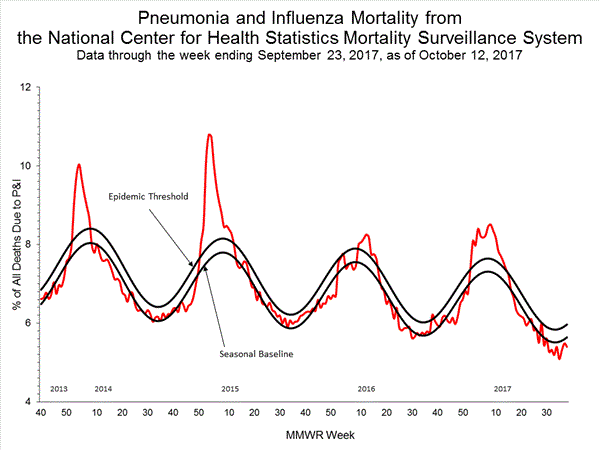
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
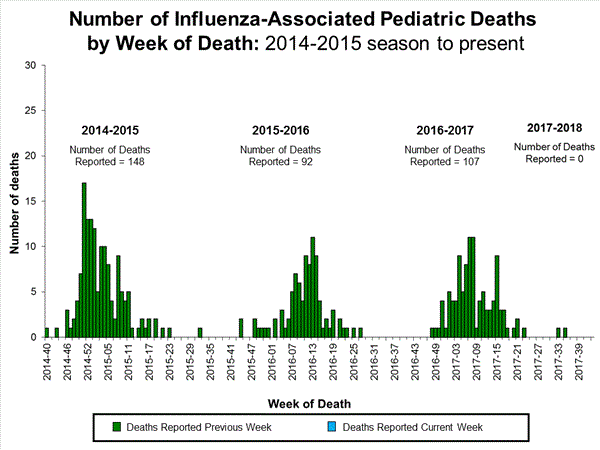
- Influenza-associated Pediatric Deaths: No influenza-associated pediatric deaths were reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 1.4%, which is below the national baseline of 2.2%. All 10 regions reported ILI below region-specific baseline levels. New York City, the District of Columbia, and 50 states experienced minimal ILI activity and Puerto Rico had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in Guam was reported as widespread; two states reported local activity; the District of Columbia and 38 states reported sporadic activity; 10 states reported no activity; and Puerto Rico and the U.S. Virgin Islands did not report.
National and Regional Summary of Select Surveillance Components
| HHS Surveillance Regions* | Data for current week | Data cumulative since October 1, 2017 (week 40) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Out-patient ILI† | Number of jurisdictions reporting regional or widespread activity§ | % respiratory specimens positive for flu in clinical laboratories‡ | A(H1N1)pdm09 | A (H3) | A (Subtyping not Performed) |
B Victoria lineage | B Yamagata lineage | B lineage not performed | Pediatric Deaths | |
| Influenza test results from public health laboratories only | ||||||||||
| Nation | Normal | 1 of 54 | 2.7% | 6 | 49 | 7 | 0 | 5 | 5 | 0 |
| Region 1 | Normal | 0 of 6 | 0.7% | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Region 2 | Normal | 0 of 4 | 1.1% | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Region 3 | Normal | 0 of 6 | 1.2% | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Region 4 | Normal | 0 of 8 | 4.5% | 5 | 9 | 1 | 0 | 0 | 1 | 0 |
| Region 5 | Normal | 0 of 6 | 1.1% | 0 | 5 | 4 | 0 | 1 | 0 | 0 |
| Region 6 | Normal | 0 of 5 | 2.1% | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Region 7 | Normal | 0 of 4 | 0.8% | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Region 8 | Normal | 0 of 6 | 1.0% | 1 | 8 | 0 | 0 | 3 | 0 | 0 |
| Region 9 | Normal | 1 of 5 | 1.4% | 0 | 17 | 0 | 0 | 0 | 4 | 0 |
| Region 10 | Normal | 0 of 4 | 1.7% | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
*https://www.hhs.gov/about/agencies/iea/regional-offices/index.html
† Elevated means the % of visits for ILI is at or above the national or region-specific baseline
§ Includes all 50 states, the District of Columbia, Guam, Puerto Rico, and U.S. Virgin Islands
‡ National data are for current week; regional data are for the most recent three weeks
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
The results of tests performed by clinical laboratories are summarized below.
| Week 40 | |
|---|---|
| No. of specimens tested | 10,152 |
| No. of positive specimens (%) | 270 (2.7%) |
| Positive specimens by type | |
| Influenza A | 182 (67.4%) |
| Influenza B | 88 (32.6%) |
View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
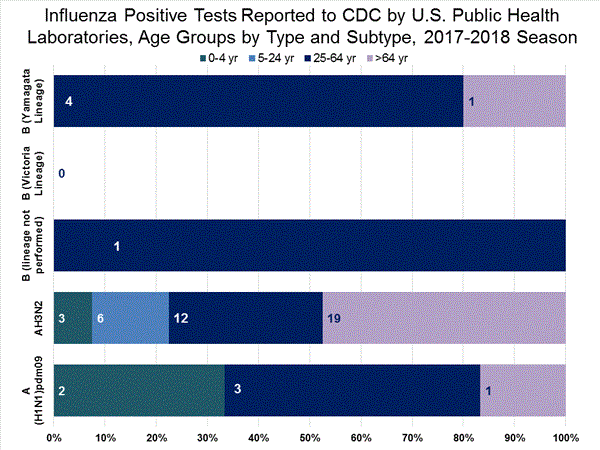
The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
| Week 40 | |
|---|---|
| No. of specimens tested | 407 |
| No. of positive specimens | 72 |
| Positive specimens by type/subtype | |
| Influenza A | 62 (86.1%) |
| A(H1N1)pdm09 | 6 (9.7%) |
| H3 | 49 (79.0%) |
| Subtyping not performed | 7 (11.3%) |
| Influenza B | 10 (13.9%) |
| Yamagata lineage | 5 (50.0%) |
| Victoria lineage | 0 (0%) |
| Lineage not performed | 5 (50.0%) |
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.

View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
View Interactive Application | View Full Screen
Influenza Virus Characterization:
CDC characterizes influenza viruses through one or more tests including genomic sequencing, hemagglutination inhibition (HI) and/or neutralization assays. These data are used to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines, and to monitor for changes in circulating influenza viruses. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine's ability to produce an immune response against the wide array of influenza viruses co-circulating, but vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses. Viruses can be classified into genetic groups/clades based on analysis of their HA gene segments using phylogenetics and key amino acid changes (Klimov Vaccine 2012).A representative subset of influenza-positive surveillance samples are antigenically characterized. However, a proportion of influenza A(H3N2) viruses lack sufficient hemagglutination titers for antigenic characterization using hemagglutination inhibition assays. Therefore, CDC selects a representative subset of influenza A(H3N2) viruses for antigenic characterization using the virus neutralization focus reduction assay to assess the ability of various antisera to neutralize infectivity of the test viruses.
It is important to monitor circulating influenza viruses for evidence of genetic changes. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in the circulating virus populations, with no evidence of substantial antigenic drift. Close monitoring of influenza viruses is required to better assess the potential impact on public health.
Genetic Characterization
During May 21 – October 7, 2017, 1,692 influenza positive specimens were collected and reported by public health laboratories in the United States (Figure, left). CDC genetically characterized 382 influenza viruses [51 influenza A(H1N1)pdm09, 230 influenza A(H3N2), and 101 influenza B viruses] collected by U.S. laboratories.
Influenza A Viruses
- A (H1N1)pdm09 [51]: The HA gene segment of all influenza A(H1N1)pdm09 viruses analyzed showed that one virus belonged to clade 6B, with the remainder belonging to 6B.1, the same genetic clade as the vaccine reference virus, A/Michigan/45/2015.
- A (H3N2) [230]: Phylogenetic analysis of the HA genes indicate that multiple clades/subclades are circulating. The HA genes show extensive diversity and belong to clades 3C.2a, subclade 3C.2a1 or 3C.3a, with 3C.2a predominating. The vaccine reference virus, A/Hong Kong/4801/2014, belongs to the genetic clade 3C.2a.
Influenza B Viruses
- B/Victoria [31]: The HA of influenza B/Victoria-lineage viruses all belonged to genetic group V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008.
- Two subgroups of viruses within V1A have been detected with a double or triple deletion of amino acids in the HA. The majority of the double deletion viruses were identified in the United States, while no triple deletion viruses have been identified in the United States
- B/Yamagata [70]: The HA of influenza B/Yamagata-lineage viruses analyzed all belonged to genetic group Y3, the same genetic clade as the vaccine reference virus, B/Phuket/3073/2013.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmap considerations, specimen submission guidance issued to the laboratories request that, if available, 2 influenza A (H1N1), 2 A influenza (H3N2), and 2 influenza B viruses be submitted every other week. Because of this, the number of each virus type/subtype characterized should be approximately equal. In the figure below, the results of tests performed by public health labs are presented on the left and sequence results by genetic group of specimens submitted to CDC are presented on the right.

View Chart Data | View Full Screen | View PowerPoint Presentation
Antigenic Characterization
During May 21 – October 7, 2017, CDC antigenically characterized 227 influenza viruses [43 influenza A(H1N1)pdm09, 116 influenza A(H3N2), and 68 influenza B viruses] collected by U.S. laboratories. Antigenic similarity is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing the recommended vaccine components of the Northern Hemisphere 2017-18 vaccine.
Influenza A Virus [159]
- A (H1N1)pdm09 [43]: All 43 influenza A(H1N1)pdm09 viruses were antigenically characterized using ferret post-infection antisera as A/Michigan/45/2015 (H1N1)pdm09-like.
- A (H3N2) [116]: 112 of 116 (96.6%) influenza A(H3N2) viruses were antigenically characterized as A/Hong Kong/4801/2014-like by HI testing or neutralization testing. Among the viruses that reacted poorly with ferret antisera raised against A/Hong Kong/4801/2014-like viruses, all belong to genetic group 3C.3a.
Influenza B Virus [68]
- Victoria Lineage [28]: 18 of 28 (64.3%) B/Victoria-lineage viruses were antigenically characterized using ferret post-infection antisera as B/Brisbane/60/2008-like. Among the viruses that reacted poorly with ferret antisera raised against B/Brisbane/60/2008-like viruses, all were double deletion viruses.
- Yamagata Lineage [40]: All 40 (100%) B/Yamagata-lineage viruses were antigenically characterized using ferret post-infection antisera as B/Phuket/3073/2013-like.
Antiviral Resistance:
No antiviral resistance data are available for specimens collected after October 1, 2017.
During May 21-Septemer 30, 2017, 364 specimens (49 influenza A(H1N1)pdm09, 218 influenza A(H3N2), and 364 influenza B viruses) collected in the United States were tested for susceptibility to the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir). All tested viruses were sensitive to all three recommended antiviral medications.
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A (H1N1)pdm09 viruses and oseltamivir-resistant influenza A (H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on October 12, 2017, 5.4% of the deaths occurring during the week ending September 23, 2017 (week 38) were due to P&I. This percentage is below the epidemic threshold of 6.0% for week 38.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. There is a backlog of data requiring manual coding within NCHS mortality surveillance data. The percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records and may result in initially reported P&I percentages that are lower than percentages calculated from final data. Efforts continue to reduce and monitor the number of records awaiting manual coding.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
View Regional and State Level Data | View Chart Data | View Full Screen | View PowerPoint Presentation
Influenza-Associated Pediatric Mortality:
No influenza-associated pediatric deaths were reported to CDC during week 40.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in the Emerging Infections Program (EIP) states and Influenza Hospitalization Surveillance Project (IHSP) states. FluSurv-NET estimated hospitalization rates will be updated weekly starting later this season.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.Outpatient Illness Surveillance:
Nationwide during week 40, 1.4% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is below the national baseline of 2.2%. (ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.

View National and Regional Level Graphs and Data | View Chart Data | View Full Screen | View PowerPoint Presentation
On a regional level, the percentage of outpatient visits for ILI ranged from 0.4% to 2.1% during week 40. All 10 regions reported a proportion of outpatient visits for ILI below their region-specific baseline levels.
ILINet State Activity Indicator Map:
Data collected in ILINet are used to produce a measure of ILI activity* by state. Activity levels are based on the percent of outpatient visits in a state due to ILI and are compared to the average percent of ILI visits that occur during weeks with little or no influenza virus circulation. Activity levels range from minimal, which would correspond to ILI activity from outpatient clinics being below, or only slightly above, the average, to high, which would correspond to ILI activity from outpatient clinics being much higher than average.
During week 40, the following ILI activity levels were experienced:
- New York City, the District of Columbia, and all 50 states experienced minimal ILI activity.
- Data were insufficient to calculate an ILI activity level from Puerto Rico.
*This map uses the proportion of outpatient visits to health care providers for ILI to measure the ILI activity level within a state. It does not, however, measure the extent of geographic spread of flu within a state. Therefore, outbreaks occurring in a single city could cause the state to display high activity levels.
Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state.
Data displayed in this map are based on data collected in ILINet, whereas the State and Territorial flu activity map is based on reports from state and territorial epidemiologists. The data presented in this map are preliminary and may change as more data are received.
Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses, but does not measure the severity of influenza activity.
During week 40, the following influenza activity was reported::
- Widespread influenza activity was reported by Guam.
- Local influenza activity was reported by two states (Colorado and South Carolina).
- Sporadic influenza activity was reported by the District of Columbia and 38 states (Alaska, Alabama, Arizona, Arkansas, California, Connecticut, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Dakota, Texas, Utah, Virginia, Washington, Wisconsin, and Wyoming).
- No activity was reported by 10 states (Delaware, Kansas, Maryland, Nebraska, Nevada, New Hampshire, Rhode Island, Tennessee, Vermont, and West Virginia,).
- Puerto Rico and the U.S. Virgin Islands did not report
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visit http://www.cdc.gov/flu/weekly/fluviewinteractive.htm.
U.S. State and local influenza surveillance: Click on a jurisdiction below to access the latest local influenza information.
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available at https://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
--------------------------------------------------------------------------------- Page last reviewed: October 13, 2017
- Page last updated: October 13, 2017
- Content source:
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD)
- Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs


 ShareCompartir
ShareCompartir