Public Health Genomics At a Glance: Identify, Inform, Integrate
OPHG provides timely and credible information for the effective and responsible translation of genome-based discoveries into public health & health care.
Identifying Opportunities for Genomics to Improve Health & Transform Health Care
Through systematic horizon scanning and evidence-based classification of genomic tests and family health history applications, OPHG is identifying those applications that are ready to impact health if implemented effectively and demonstrating how the genomic transformation of health care is moving from promise to reality. OPHG developed, maintains and updates the Genomic Applications in Practice and Prevention Knowledge Base, an on-line database that captures emerging genomic tests with the potential to impact public health, which currently includes over 540 genomic tests.
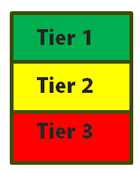
In 2012, OPHG developed a three-tiered framework for classifying genomic testing and family health history applications based on the availability of scientific evidence and evidence-based recommendations supporting their use. Tier 1/green applications are supported by a base of synthesized evidence for implementation in practice. Tier 2/yellow applications may provide information for informed decision making based on existing evidence; however, synthesized evidence is insufficient to support routine implementation in practice. Tier 3/red applications are not ready for routine implementation in practice based on synthesized evidence culminating in recommendations against use, or no relevant synthesized evidence identified; however, they might be candidates for population or clinical research.
To date, OPHG has identified 49 tier 1/green genomic applications supported by evidence for use in clinical and/or public health practice.
Since 2013, with the increasing applications of next generation sequencing in healthcare, OPHG has promoted an evidence based approach for the use of the technology in sick individuals and healthy populations. In addition, OPHG promoted an ethical and evidentiary framework for the handling of incidental findings in clinical sequencing studies.

Evaluating genomic applications
Since 2004, OPHG has supported the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group; an independent, nonfederal, multidisciplinary panel that systematically reviews evidence on emerging genomic applications in clinical practice and disease prevention and produces recommendations for their use. To date, there have been 13 EGAPP-sponsored reports and evidence reviews and 10 EGAPP recommendation statements. EGAPP recommendations have led to appropriate use of new technologies in saving lives and reducing unnecessary healthcare costs. More than 90 institutions have used the 2009 EGAPP Lynch syndrome recommendation to justify Lynch syndrome screening protocols. The EGAPP working group has produced recommendations against or discouraging the routine use of three genetic tests (factor V Leiden in recurrence of venous thrombosis, cardiogenomic profiles in risk assessment for heart disease, and CYP450 for selection and dosage of selective serotonin reuptake inhibitors in major depression), thereby helping to reduce unnecessary health care costs and potential harms to patients. The Knowledge Synthesis Center of the EGAPP working group has developed and applied a new methodology for classifying genetic variants by clinical actionability in next-generation sequencing studies.
In 2015, OPHG and partners will transition toward a new Genomics and Population Health Action Collaborative to explore public health approaches for accelerating the translation of evidence-based genomic tests and family health history into population health benefits.
Informing About Evidence-based Genomic Applications to Impact Health
OPHG has developed a multifaceted communication strategy, including the OPHG website, Genomics and Health Impact Update and blog, tweets, podcasts and reports, to share information on genomics and health.
OPHG’s Genomics and Health Impact Update is published weekly, providing timely information about genomic tests, family health history, and relevant policy and legislation, science, and practice to over 66,000 subscribers.
OPHG’s Genomics and Health Impact blog addresses current topics such as family health history, direct-to-consumer personal genomic tests, preparedness, smoking, pathogen and human genomics, with over 155,000 views.
OPHG has reached over 30,000 viewers with evidence-based genomics podcasts, including a new CDC Expert Commentary Series on Medscape released in 2014.
@DrKhouryCDC has over 8,000 followers and tweets frequently on translating genome-based discoveries into actions that improve health & prevent disease.
OPHG is the lead editor for the online collection PLoS Currents: Evidence on Genomic Tests, which includes evidence summaries for 36 genomic tests to date.
Integrating Evidence-based Genomic Applications into Practice & Programs
Nearly 2 million Americans are at increased risk for early-onset cancer or heart disease because they have one of three genetic conditions: BRCA-associated hereditary breast and ovarian cancer, Lynch syndrome, and familial hypercholesterolemia. Most people with these conditions are not aware they have them, yet early detection and intervention could save their lives. Cost-effective, evidence-based interventions exist to prevent premature disease and death from heart disease and cancer in people with these conditions. Among the tier 1/green genomic applications identified to date, OPHG has focused integration efforts on these three genomic applications with evidence-based recommendations to support their use in healthcare and public health.

State public health departments are reaching people with these conditions by integrating evidence-based detection and referral into existing public health programs.
Model state genomics programs are:
- Identifying people eligible for recommended genomic services using cancer registries, and educating health providers.
- Facilitating payer coverage consistent with evidence-based recommendations by implementing model policies.
- Monitoring implementation of genomics recommendations by developing and evaluating new data sources.
Examples of successful state efforts include:
- Michigan Department of Community Health (MDCH) identified more than 15,000 people who might benefit from genetic evaluation for hereditary breast, ovarian, colorectal and other cancers, using existing cancer registry data (2006-2007).
- MDCH has partnered with payers using a model policy implementation program to extend insurance coverage to over 6 million Michigan residents for genetic services consistent with U.S. Preventive Services Task Force BRCA recommendations.
These successes highlight what might be possible on a national scale.
In 2014, OPHG released a Tier 1 Genomic Applications Toolkit and several video resources, including Public Health Genomics Implementation to Save Lives – From National Vision to State Success featuring prominent public health and health care provider, payer, and patient leaders. The toolkit includes case studies describing successful state efforts with links to state resources.
In 2015, OPHG will release new updates to the toolkit, including resources to help states use cancer registry data to identify people at risk for hereditary cancer, as well as program and policy considerations for states interested in promoting cascade screening in families at risk for hereditary forms of cancer and heart disease.
In 2013, OPHG released a clickable state map compiling state resources and activities relevant to the implementation of evidence-based recommendations for BRCA-associated hereditary breast and ovarian cancer, Lynch syndrome, and familial hypercholesterolemia.
These new resources were developed to help states and their partners build upon existing programs to reach people with these three conditions, and they serve as a framework for incorporating additional tier 1 genomic applications in the future.
- Page last reviewed: June 12, 2017
- Page last updated: June 20, 2017
- Content source:


 ShareCompartir
ShareCompartir