ACCE Model Process for Evaluating Genetic Tests
This website is archived for historical purposes and is no longer being maintained or updated.
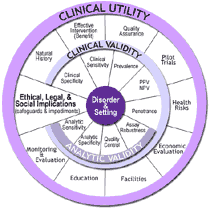
From 2000 – 2004, CDC’s Office of Public Health Genomics (OPHG) established and supported the ACCE Model Project, which developed the first publicly-available analytical process for evaluating scientific data on emerging genetic tests. The ACCE framework has guided or been adopted by various entities in the United States and worldwide for evaluating genetic tests; the CDC-supported EGAPP™ initiative builds on the ACCE model structure and experience.
Introduction to ACCE
ACCE, which takes its name from the four main criteria for evaluating a genetic test — analytic validity, clinical validity, clinical utility and associated ethical, legal and social implications — is a model process that includes collecting, evaluating, interpreting, and reporting data about DNA (and related) testing for disorders with a genetic component in a format that allows policy makers to have access to up-to-date and reliable information for decision making. The ACCE model process is composed of a standard set of 44 targeted questions (3) that address disorder, testing, and clinical scenarios, as well as analytic and clinical validity, clinical utility, and associated ethical, legal, and social issues.
An important by-product of the ACCE model process is the identification of gaps in knowledge that will help to define future research agendas. The ACCE approach builds on a methodology originally described by Wald and Cuckle (1) and on terminology introduced by the Secretary’s Advisory Committee on Genetic Testing (2).
Learn more about ACCE.
ACCE Resources
-
ACCE
Model List of 44 Targeted Questions - First ACCE Review: Population-based Prenatal Screening for Cystic Fibrosis via Carrier Testing
References
- Wald N, Cuckle H. Reporting the assessment of screening and diagnostic tests. Br J Obstet Gynaecol 1989 Apr;96(4):389-96.
- Department of Health and Human Services, Secretary’s Advisory Committee on Genetic Testing. Request for public comment on a proposed classification methodology for determining level of review for genetic tests. Federal Register 2000;65(236):76643-76645.
- Haddow JE, Palomaki GE. ACCE: A Model Process for Evaluating Data on Emerging Genetic Tests. In: Human Genome Epidemiology: A Scientific Foundation for Using Genetic Information to Improve Health and Prevent Disease. Khoury M, Little J, Burke W (eds.), Oxford University Press, pp. 217-233, 2003.
- Burke W, Zimmern R. Moving Beyond ACCE: An Expanded Framework for Genetic Test Evaluation [344 KB]. A paper for the United Kingdom Genetic Testing Registry, PHG Foundation, 2007.
- Page last reviewed: December 28, 2010 (archived document)
- Content source:


 ShareCompartir
ShareCompartir