Multistate Outbreak of Fungal Meningitis and Other Infections –Resources for Laboratories
On October 30, 2015, CDC updated its web resources for patients and clinicians. Patients affected by tainted steroid injections from the New England Compounding Center continue to receive treatment for their infections and clinicians should continue to monitor patient recovery. All relevant materials for patients and clinicians concerning the multistate outbreak of fungal meningitis and other infections are located on this page.
Testing and Results
CDC Laboratory Results
CDC laboratory-confirmed results found on this page are from three New England Compounding Center (NECC) preservative-free methylprednisolone acetate (MPA) lots recalled on September 26, 2012. [1]
For laboratory results on NECC products other than preservative-free MPA, see CDC’s non-MPA recall website and FDA’s website.
CDC’s Fungus Reference Laboratory has the capacity and technology to examine fungal isolates under the microscope and to confirm their identification using DNA sequencing methods.
Human Tissue and Fluid Samples [2, 3, 4]
Since the start of the outbreak in September 2012, CDC and State health laboratories have tested tissue and fluid samples from patients with probable or confirmed fungal infection. CDC scientists used DNA-based methods and other advanced testing to determine whether the samples contain fungi and other microbial pathogens, and if so, what type.
As of March 11, 2013, Exserohilum rostratum has been identified as the predominant fungal infection in this outbreak. One patient, the index case, was infected with Aspergillus fumigatus, and 22 additional species were identified: Aspergillus species (4 cases), Cladosporium spp. (6), Alternaria sp. (4), Bipolaris sp. (1), Chaetomium sp. (1), coelomycete fungus (1), Epicoccum nigrum (1), Paecilomyces (1), Penicillium sp. (1), Scopulariopsis brevicaulis (1), and Stachybotrys chartarum (1).
Early in the outbreak, patient specimens were primarily cerebrospinal fluid and fluid from joints (knee, etc.). As the outbreak progressed, fluids from epidural abscesses (accumulations of pus around the spine) and tissues became the focus of laboratory testing.
The numbers reported here do not reflect all cases of fungal meningitis, joint infections, and epidural abscesses because laboratory methods are not sensitive enough to detect all positive samples. Many patients with potential fungal infections will have a negative laboratory result. [2, 3]
Table 1
| Source | Number of E. rostratum Identified | Fungus Description |
|---|---|---|
| Cerebrospinal Fluid | 82 | Exserohilum is a common mold found in soil and on plants, especially grasses, and thrives in warm and humid climates. Exserohilum rarely causes infections in people, however Exserohilum rostratum has been recognized as a human pathogen. More |
| Tissue | 48 | |
| Joint fluids | 4 | |
| Other specimens | 2 | |
| Cultured Isolates† | 49 |
* Multiple specimens were received from some case-patients
† Fungal cultures isolated from human specimens that were received from State Health Department laboratories.

Photo 1
Antifungal Susceptibility Testing [5]
Fifty isolates of Exserohilum rostratum were cultured from patient specimens and tested for their susceptibility to a variety of antifungal drugs. Susceptibility to amphotericin B was determined using the Epsilometer test (Etest) method according to the manufacturer’s instructions [Photo 1]. Susceptibility to voriconazole, posaconazole, itraconazole, fluconazole, and the investigational drug isavuconazole was tested by broth microdilution according to methods defined by the Clinical and Laboratory Standards Institute (CLSI) (M38-A2) [5]. Exserohilum rostratum drug susceptibility results are shown in Table 2 below.
Table 2
| Minimum Inhibitory Concentration (in µg/ml) at 48-72h | ||||
|---|---|---|---|---|
| Antifungal agent (no. of isolates tested) | Range (lowest to highest value) | MIC50 | MIC90 | Mode (most frequent value) |
| Voriconazole (50) | 1-4 | 1 | 2 | 1-2 |
| Fluconazole (50) | 16-256 | 64 | 64 | 64 |
| Itraconazole (50) | 0.25-4 | 0.5 | 1 | 0.5 |
| Posaconazole (49) | 0.25-1 | 0.5 | 1 | 0.5 |
| Isavuconazole (50) | 2-4 | 4 | 4 | 4 |
| Amphotericin B (48) | 0.032-2 | 0.25 | 0.5 | 0.38 |
The clinical relevance of MIC testing of this fungal pathogen remains uncertain, and breakpoints with proven relevance have yet to be identified or approved by CLSI or any regulatory agency.
CDC's PCR test for detection of fungal DNA
CDC’s fungal PCR test at a glance
- Limit of detection: 10 copies of DNA per reaction
- Diagnostic sensitivity: 47%
- Diagnostic specificity: 100
Once the primary pathogen in this outbreak was determined to be Exserohilum rostratum, CDC developed a real-time PCR assay to detect DNA from this organism in cerebrospinal fluid (CSF) and other body fluids from patients in this outbreak. To assess the performance of this PCR test, CDC tested 888 samples from 461 case-patients. The main results of the validation study are as follows:
- The real-time PCR test detected Exserohilum rostratum DNA in 209 samples from 171 case-patients (37% of the 461 case-patients for whom samples were available).
- Real-time PCR was more sensitive than either culture or the CDC conventional PCR assay. Of 139 case-patients who had a specimen tested by real-time PCR, CDC conventional PCR and culture, E. rostratum DNA was detected by real-time PCR in 65 (47%) specimens, by conventional PCR in 41 (29%), and was recovered from culture in 19 (14%). Among 136 specimens from patients who did not meet the case definition, all were negative. All samples positive in the conventional PCR assay were also positive in the real-time assay, and five samples that were positive in culture but negative in the conventional PCR assay were positive in the real-time assay.
- A low level of amplification (Ct value of 42 or 43) was detected in 7 of 61 water blank samples, indicating contamination during extraction.
The full results of the study performed to validate this real-time PCR test are available here [PDF – 739 KB]. The results suggest that the real-time PCR test is a useful tool to quickly detect fungi in body fluids and tissues, and it can detect fungal DNA in specimens that may not have shown any fungus in culture. This assay is specific for Exserohilum rostratum and cannot be used to detect other fungi. This assay is very sensitive to contamination and should be used only in conjunction with epidemiologic and clinical data.
However, PCR for fungal detection is still a research test that has not been cleared or approved by the FDA and should not be used for diagnosis, treatment, or assessment of patient health or management. In addition, for both PCR and culture, a negative result does not rule out infection.
Beta-D-glucan testing for diagnostics and monitoring response to treatment
Beta-D-glucan testing at a glance
- Specimen tested: CSF (cerebrospinal fluid)
- Test used: Fungitell test, Associates of Cape Cod, Falmouth MA
- Diagnostic sensitivity: 100% at a cutoff value of 138 picograms/ml
- Diagnostic specificity: 98% at a cutoff value of 138 picograms/ml
CDC assessed the value of the Fungitell beta-D-glucan (BDG) test for diagnostics and for monitoring response to treatment of patients in this outbreak. To test diagnostics, we evaluated 108 incident CSF samples from proven or probable case patients. Of 41 samples from proven cases, BDG levels of 40 samples were >230 picograms/ml, and 1 was 205 pg/ml. Of 67 samples from probable cases, BDG levels were <230 pg/ml for 30 samples, 139-195 pg/ml for 2 samples, and <138 pg/ml for 35 samples. To test the value of the BDG assay in monitoring response to treatment, BDG levels were assessed in serial CSF samples collected from 20 patients. We found that among patients in whom CSF BDG levels progressively declined, most remained asymptomatic after completing antifungal therapy. Patients in whom CSF BDG levels remained persistently high had poor outcomes, although the number of patients in this group was small.
The full results of the study performed to validate this Beta-D-glucan test are available here. The results of this investigation suggest that the beta-D-glucan test may be a useful tool to detect fungal cell wall components in cerebrospinal fluid (CSF). However, Beta-D-glucan measurement in CSF for fungal detection is still a research test that has not been cleared or approved by the FDA for making decisions regarding the clinical management of patients. In addition, for both PCR and BDG, a negative result does not rule out infection.
Product Samples: Three Lots of Preservative-Free MPA
Tests at CDC and FDA laboratories on the preservative-free MPA vials have confirmed the presence of Exserohilum rostratum in unopened vials from two of the three recalled lots. [1] These laboratory test results strengthen the link between preservative-free MPA vials and the outbreak.
The types of fungi found in patient specimens and in the recalled MPA vials are common in the environment, but were not known to cause meningitis prior to this outbreak.
- As of October 22, CDC laboratories have confirmed the presence of Exserohilum rostratum [JPG] in lots #08102012@51 BUD 2/6/2013 and #06292012@26 BUD 12/26/2012. This fungus is the same genus and species as the one found in laboratory-confirmed cases of human infection.
- As of January 22, 2013, CDC laboratories have recovered Cladosporium cladosporioides in one unopened vial from lot#0812012@51 sent from the state of Tennessee. This fungus has also been recovered from five patient isolates sent to CDC for confirmation, and from one CSF sample sent to CDC for testing.
- As of February 8, 2013, CDC laboratories confirmed the presence of Exserohilum rostratum, Bacillus subtilis and Bacillus pumilus in one unopened vial of lot 08102012@51 that was cultured locally in Idaho and submitted to CDC through the Idaho state public health laboratory. The Bacillus organisms are bacteria.
- As of February 8, 2013, CDC laboratories reconfirmed Paecilomyces formosus from 20 unopened vials from lot 05212012@68 cultured and identified at the Wadsworth Center New York State public health laboratory. The New York State laboratory also reported the detection of Exserohilum rostratum from one unopened vial of this lot, using a real-time PCR assay developed in their laboratory.
- As of May 19, 2015, CDC laboratories recovered Methylobacterium sp. bacteria in unopened vials from lot 05212012@68.
- CDC also identified non-human pathogens Rhodotorula laryngis and Rhizopus stolonifer in lot #08102012@51, BUD 2/6/2013; and Rhodotorula laryngis in lot #06292012@26, BUD 12/26/2012. The fungi Rhodotorula laryngis and Rhizopus stolonifer are not known to cause disease in humans; they cannot grow at human body temperature. CDC also identified Methylobacterium sp. bacteria in lot 06292012@26.
Table 3
| Product(s) | Fungi Isolated | Description |
|---|---|---|
|
MPA (PF) Lot #06292012@26 MPA (PF) Lot #08102012@51 |
Exserohilum rostratum | Known to cause human disease |
|
MPA (PF) Lot #08102012@51 |
Cladosporium cladosporioides | Does not grow at human body temperature |
|
MPA (PF) Lot #06292012@26 MPA (PF) Lot #08102012@51 |
Rhodotorula laryngis |
Does not grow at human body temperature Not known to cause human disease |
| MPA (PF) Lot #08102012@51 Beyond Use Date 2/6/2013 |
Rhizopus stolonifer |
Does not grow at human body temperature Not known to cause human disease |
- Three NECC MPA (PF) 80mg/ml lots recalled on September 26, 2012
MPA (PF) 80 mg/ml Injection, Lot #05212012@68, Beyond Use Date 11/17/2012
MPA (PF) 80 mg/ml Injection, Lot #06292012@26, Beyond Use Date 12/26/2012
MPA (PF) 80 mg/ml Injection, Lot #08102012@51, Beyond Use Date 2/6/2013 - Establishing a definitive diagnosis of fungal meningitis caused by molds is challenging because of the low sensitivity of culture and microscopic examination.
- CDC has conducted laboratory testing to compare the Aspergillus fumigatus strain isolated from the case-patient in Tennessee with the strain isolated from the heart transplant patient in Massachusetts who was exposed to NECC-cardioplegia solution that was recalled on October 6, 2012. These two strains did not match and were shown to be different from each other, providing evidence against a common source of Aspergillus fumigatus for the Tennessee patient who was exposed to the NECC preservative-free MPA and the Massachusetts patient who was exposed to the NECC-cardioplegia solution
- A negative fungal polymerase chain reaction (PCR) test from a lumbar-puncture diagnostic specimen does not rule out fungal infection. Active fungal infection may be present even when these tests are negative. We do not expect that growth in culture results or PCR tests will definitively identify all fungal pathogens in all cases, and infection with multiple fungi is possible. In treating patients with presumed fungal infection associated with this outbreak, CDC recommends following its treatment guidance even in cases in which testing fails to reveal one or more fungal pathogens. Early initiation and prolonged continuation of therapy may improve clinical outcome. Therefore, therapy should not be withheld or discontinued in the absence of laboratory confirmation in such patients (See Wijeyaratne and Harshalal, Aspergillus meningitis in Sri Lanka).
- Clinical and Laboratory Standards Institute (CLSI). 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard – Second Edition. CLSI Document M38-A2. Wayne, PA: Clinical and Laboratory Standards Institute.
Pathologic Evaluation
Specimen Submission Guidelines
Download Specimen Submission Guidelines for Pathologic Evaluation of CNS Infections [PDF – 1 page]
Histopathologic changes in tissues and the pathogens that cause acute meningitis or encephalomyelitis may be distributed focally and sparsely in the central nervous system, and the predilection site for infection may vary among different organisms. Collecting multiple representative portions of central nervous system (CNS) tissue, as well as tissue samples from any other organ system with inflammatory cell infiltrates, ensures the best chance of detecting the causative agent. Performance of specific immunohistochemical, molecular, or other assays will be determined using clinical and epidemiologic information provided by the submitter and the histopathologic features identified in the submitted tissue specimens.
Collection of Tissue Specimens
As part of this outbreak, the preferred specimens include paraffin blocks of involved CNS tissue, or representative tissues in formalin (i.e., wet tissue). Fresh-frozen tissue may also be submitted for culture and molecular-based assays. Representative tissue specimens from each of the following sites should be obtained and submitted for evaluation:
- Cerebral cortex (frontal, parietal, temporal, and occipital)
- Brain stem (midbrain, pons, medulla) and spinal cord
- Cerebellum
- Basal ganglia, thalamus, hypothalamus, and hippocampus
- Meninges
Submission of Specimens
Paraffin-embedded tissue blocks
In general, this type of specimen is the preferred and is especially important to submit in cases where tissues have been in formalin for a significant time. Prolonged fixation (>2 weeks) may interfere with some immunohistochemical and molecular diagnostic assays.
Wet tissue
If available, we highly recommend that unprocessed tissues in 10% neutral buffered formalin be submitted in addition to paraffin blocks.
Unstained slides
Although not optimal, if paraffin blocks are unavailable it may be possible to use unstained sections cut at 3–5 microns (10 slides per block) for immunohistochemistry and specials stains but not molecular diagnostic assays (e.g., PCR).
Fresh-frozen tissue
Send separately on dry ice.
Electron Microscopy (EM) specimens
Samples fixed in glutaraldehyde and held in phosphate buffer. Sample containers are filled to the top with phosphate buffer and sent on wet ice. Do not freeze. Epoxy-embedded tissues are also accepted.
Specimen shipping information: Please contact your state health department and state public health laboratory to coordinate shipment of specimens to CDC for further testing.
Pathologic Results
Microscopic Gallery
Information on this site is focused on important laboratory findings and guidance related to the outbreak of fungal meningitis and other infections linked to the use of injectable steroids from three recalled lots of preservative-free methylprednisolone acetate (MPA) distributed by the New England Compounding Center (NECC).
Disease Caused by Fungus in Brain Tissue

Major histopathologic findings in clinical cases of meningitis show evidence of necrotizing, suppurative vasculitis with thrombosis (A and B). These findings are seen in many cases involving a branch of the basilar artery (C), which are consistent with the clinical findings.
Disease Caused by Fungus in Brain Tissue

Another finding in the basilar artery is necrosis (A) and giant cell arteritis (B).
Fungus in Tissue Surrounding the Spinal Cord
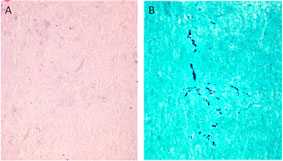
Arachnoiditis is a complication present in some of the patients. In tissue obtained adjacent to the affected site, dense collagen-rich soft tissue with no inflammatory infiltrate (A) is seen. Fungal hyphae are seen in this tissue by silver stain (GMS) with modified oxidation times (B).
Fungus in a Hip Joint
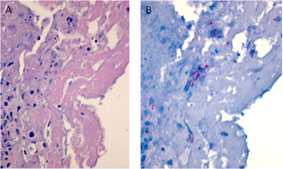
In patients with arthritis, fibrin and necrotic debris are seen in affected joints (A). Although rare, immunohistochemistry facilitates the focal detection of hyphae (B) in these areas (red staining).
Fungus in Brain Tissue
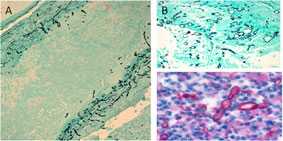
Fungal hyphae (black) can be visualized with silver stain within vessel walls (A) and in area of necrosis in basilar artery (B). Using a polyfungal immunohistochemistry reagent, fungal hyphae (red) is also seen in the purulent exudate in spinal meningitis (C).
Additional Resources
CDC Morbidity and Mortality Weekly Report (MMWR)
- MMWR: Multistate Outbreak of Fungal Infection Associated with Injection of Methylprednisolone Acetate Solution from a Single Compounding Pharmacy — United States, 2012
- MMWR: Multistate Fungal Meningitis Outbreak — Interim Guidance for Treatment
CDC Resources
- Frequently Asked Questions for Patients
- CDC Digital Press Kit with Laboratory Photos and Footage
- CDC Fungal Diseases
- CDC Fungal Meningitis
- CDC Information About Additional Medical Products (non-MPA) From New England Compounding Center
- CDC Information About Voluntary Recall of All Ameridose Medical Products
Select Publications
- Gade L, Grgurich DE, Kerkering TM, Brandt ME, Litvintseva AP. Utility of Real-Time PCR for Detection of Exserohilum rostratum in Body and Tissue Fluids during the Multistate Outbreak of Fungal Meningitis and Other Infections. J Clin Microbiol 2015 Feb.
- Gade L, Scheel CM, Pham CD, et al. Detection of fungal DNA in human body fluids and tissues during a multistate outbreak of fungal meningitis and other infections. Eukaryotic Cell 2013 Mar.
- Kainer MA, Reagan DR, Nguyen DB, et al. Fungal infections associated with contaminated methylprednisolone in Tennessee. New Engl J Med 2012 Nov.
- Litvintseva AP, Hurst S, Gade L, et al. Whole-genome analysis of Exserohilum rostratum from an outbreak of fungal meningitis and other infections. J Clin Microbiol 2014 Sep.
- Litvintseva AP, Lindsley MD, Gade L, et al. Utility of (1–3)-β-d-Glucan Testing for Diagnostics and Monitoring Response to Treatment During the Multistate Outbreak of Fungal Meningitis and Other Infections. Clin Infect Dis 2013 Dec.
- Lockhart SR, Pham CD, Gade L, et al. Preliminary Laboratory Report of Fungal Infections Associated with Contaminated Methylprednisolone Injections. J Clin Microbiol 2013 Aug.
- Lyons JL, Gireesh ED, Trivedi JB, et al. Fatal Exserohilum meningitis and central nervous system vasculitis after cervical epidural methylprednisolone injection. Ann Intern Med 2012 Oct.
- Pettit AC, Kropski JA, Castilho JL, et al. The index case for the fungal meningitis outbreak in the United States. NEJM 2012 Oct.
U.S. Food and Drug Administration (FDA)
- Page last reviewed: October 29, 2015
- Page last updated: October 29, 2015
- Content source:


 ShareCompartir
ShareCompartir