LSP: Lipids Standardization Program
The Lipids Standardization Program (LSP) monitors the accuracy of research and clinical laboratories over time. LSP ensures the analytical accuracy and precision of measurements performed in research studies and routine clinical laboratories by providing blinded standards (LSP samples) traceable to the CDC Reference Laboratory for measuring total cholesterol (TC), glycerides (TG), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A-I (apo A-I), and apolipoprotein B (apo B). The LSP is unique among external quality-control systems (EQAS) because it provides a way to establish, assess, and improve the analytical accuracy and precision measurements over time.
LSP participants (clinical and research laboratories) report their measurement results from the LSP samples to CDC where results are evaluated. Those participants meeting defined performance criteria for accuracy and precision receive a certificate of performance and are considered CDC-certified.
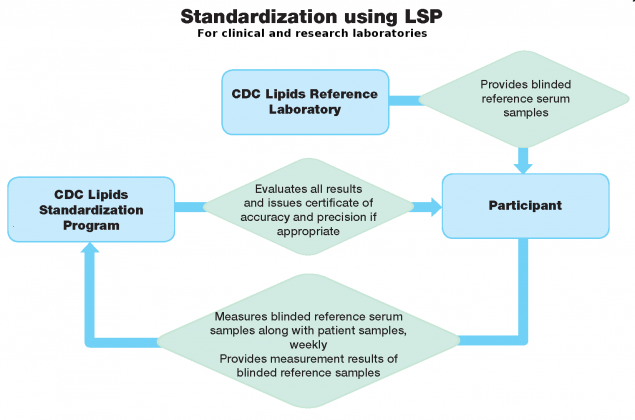
What does LSP do?
LSP monitors the accuracy and precision of its laboratory participants over time by collecting weekly measurement results from sera provided to them. It uses statistical procedures to evaluate measurement accuracy and precision over a period of three months, and issues quarterly reports to its participants.
What do LSP participants do?
LSP participants analyze three sera samples provided to them weekly, for 12 weeks. Participants are unaware of each sample’s lipids concentrations. These samples must be included in routine analytical runs, along with clinical samples. Participants receive new sets of blinded reference serum samples to be analyzed every 12 weeks (four sets per year), and report measurement results to CDC.
What are CDC’s responsibilities in the LSP process?
CDC provides participants with a statistical report that evaluates the accuracy and precision of their analyses. CDC issues a certificate of accuracy and precision if established analytical performance criteria are met.
What happens when participants do not meet performance criteria?
If performance criteria are not met, the participant should take appropriate corrective actions before proceeding with subsequent surveys. Guidelines and instructions are provided to all participants about conducting measurements and using the LSP Laboratory Data Collection System on our secure website.
What type of samples are used in LSP?
The LSP program uses unaltered, high quality pooled sera for evaluating the analytical performance of its laboratory participants. These sera are very similar to regular patient samples.
How are reference values assigned in LSP?
Reference values for TC, TG, HDL-C are assigned by the CDC Lipids Reference Laboratory. Reference values for apo A-I, and apo B are assigned outside of CDC, by the Northwest Lipid Metabolism and Diabetes Research Laboratories (NWLMDRL).
What makes LSP unique?
LSP is a unique program, because it evaluates measurement accuracy and precision over a period of three months. Other programs, such as External Quality Assessment/Proficiency Testing Program (EQA/PT), assess analytical measurement performance at a particular point in time without taking previous measurement results into account.
How can my laboratory enroll in LSP?
Please contact us at cdclsp@cdc.gov to request an application packet and submit the completed application for approval.
What are acceptable performance criteria in LSP?
Criteria for Acceptable Performance for the
CDC Lipids Standardization Program (expressed in mg/dL)
| Analyte | Concentration Range | Maximum Allowable Bias | Maximum Allowable Standard Deviation |
|---|---|---|---|
| HDL-C | <40.0 ≥40.0 |
0.05 (RV) 0.05 (RV) |
1.7 0.04 (RV) |
| TC | 100-149.9 > 149.9 |
0.03 (RV) 0.03 (RV) |
4.0 0.03 |
| TG | 0.0-88.0 > 88-176.0 > 176.0-220.0 > 220 |
9 10 11 0.05 (RV) |
7 8 10 0.05 (RV) |
Criteria for Acceptable Performance for the
CDC Lipids Standardization Program (expressed in mmol/L)
| Analyte | Concentration Range | Maximum Allowable Bias | Maximum Allowable Standard Deviation |
|---|---|---|---|
| HDL-C (mmol/L) | <1.03 ≥1.03 |
0.05 (RV) 0.05 (RV) |
0.0440 0.04 (RV) |
| TC (mmol/L) | 2.586-3.877 > 3.877 |
0.03 (RV) 0.03 (RV) |
0.103 0.03 (RV) |
| TG (mmol/L) | 0.00-0.994 > 0.994-1.989 > 1.989-2.486 > 2.486 |
0.102 0.113 0.124 0.05 (RV) |
0.079 0.090 0.113 0.05 (RV) |
There are no established criteria for acceptable performance for apolipoproteins A-I and B.
TC: Total Cholesterol
HDL-C: High Density Lipoprotein Cholesterol
TG: Total Glycerides
- Page last reviewed: July 6, 2017
- Page last updated: July 6, 2017
- Content source:


 ShareCompartir
ShareCompartir
