 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Malaria Surveillance -- United States, 1994S. Patrick Kachur, M.D., M.P.H., Megan E. Reller, M.D.C.M., Ann M. Barber, Lawrence M. Barat, M.D., M.P.H., Emilia H.A. Koumans, M.D., Monica E. Parise, M.D., Jacqueline Roberts, M.S., Trenton K. Ruebush II, M.D., Jane R. Zucker, M.D., M.Sc. Division of Parasitic Diseases National Center for Infectious Diseases Abstract Problem/Condition: Malaria is caused by infection with one of four species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae), which are transmitted by the bite of an infective female Anopheles sp. mosquito. Most malarial infections in the United States occur in persons who have traveled to areas (i.e., other countries) in which disease transmission is ongoing. However, cases are transmitted occasionally through exposure to infected blood products, by congenital transmission, or by local mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to adapt prevention recommendations. Reporting Period Covered: Cases with onset of symptoms during 1994. Description of System: Malaria cases confirmed by blood smear are reported to local and/or state health departments by health-care providers and/or laboratories. Case investigations are conducted by local and/or state health departments, and the reports are transmitted to CDC through the National Malaria Surveillance System (NMSS), which was the source of data for this report. Numbers of cases reported through NMSS may differ from those reported through other passive surveillance systems because of differences in the collection and transmission of data. Results: CDC received reports of 1,014 cases of malaria with onset of symptoms during 1994 among persons in the United States or one of its territories. This number represented a 20% decrease from the 1,275 cases reported for 1993. P. vivax, P. falciparum, P. malariae, and P. ovale accounted for 44%, 44%, 4%, and 3% of cases, respectively. More than one species was present in five persons (less than 1% of the total number of patients). The infecting species was not determined in 50 (5%) cases. The number of reported malaria cases in U.S. military personnel decreased by 86% (i.e., from 278 cases in 1993 to 38 cases in 1994). Of the U.S. civilians who acquired malaria during travel to foreign countries, 18% had followed a chemoprophylactic drug regimen recommended by CDC for the area to which they had traveled. Five persons became infected while in the United States; the infection was transmitted to two of these persons through transfusion of infected blood products. The remaining three cases, which occurred in Houston, Texas, were probably locally acquired mosquitoborne infections. Four deaths were attributed to malaria. Interpretation: The 20% decrease in the number of malaria cases from 1993 to 1994 resulted primarily from an 86% decrease in cases among U.S. military personnel after withdrawal from Somalia. Because most malaria cases acquired in Somalia during 1993 resulted from infection with P. vivax, there was a proportionately greater decrease during 1994 in the number of cases caused by P. vivax relative to those caused by P. falciparum. Actions Taken: Additional information was obtained concerning the four fatal cases and the five cases acquired in the United States. Malaria prevention guidelines were updated and distributed to health-care providers. Persons traveling to a geographic area in which malaria is endemic should take the recommended chemoprophylactic regimen and should use protective measures to prevent mosquito bites. Persons who have a fever or influenza-like illness after returning from a malarious area should seek medical care; medical evaluation should include a blood smear examination for malaria. Malarial infections can be fatal if not promptly diagnosed and treated. Recommendations concerning prevention and treatment of malaria can be obtained from CDC. INTRODUCTION Malaria is caused by infection with any of four species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae). The infection is transmitted by the bite of an infective female Anopheles sp. mosquito. Forty percent of the world's population live in areas where malaria is transmitted (e.g., parts of Africa, Asia, Central America, Hispaniola, North America, Oceania, and South America). In the past, malaria was endemic throughout much of the continental United States; an estimated 600,000 cases occurred during 1914 (1). During the late 1940s, a combination of improved socioeconomic conditions, water management, vector-control efforts, and case management was successful at interrupting malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission. Through 1994, almost all cases of malaria diagnosed in the United States were imported from regions of the world where malaria transmission occurred. Each year, however, several congenital infections and infections resulting from exposure to blood or blood products have been reported in the United States. In addition, limited numbers of cases have been reported that may have been acquired through local mosquitoborne transmission (2). State and/or local health departments and CDC thoroughly investigate all malaria cases acquired in the United States, and CDC conducts an analysis of all imported cases to detect trends in acquisition. This information is used to adapt malaria prevention recommendations for travelers abroad. For example, an increase in P. falciparum malaria among U.S. travelers to Africa, an area with increasing incidence of chloroquine resistance, prompted CDC in 1990 to change the recommended chemoprophylaxis from chloroquine to mefloquine (3). The signs and symptoms of malaria are variable, but most patients have fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should be considered for any person who has these symptoms and who has traveled to an area in which malaria is transmitted. Malaria also should be considered in the differential diagnosis of illness in persons who have a fever of unknown origin, regardless of their travel history. Untreated P. falciparum infections can progress to coma, renal failure, pulmonary edema, and death. Asymptomatic parasitemia can occur among long-term residents of malaria-endemic areas. This report summarizes malaria cases with symptom onset in 1994 reported to CDC through the National Malaria Surveillance System (NMSS). METHODS Sources of Data Data regarding malaria cases are reported to both NMSS and the National Notifiable Diseases Surveillance System. Although both systems rely on passive reporting, the numbers of reported cases may differ because of differences in the collection and transmission of data. In addition, NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to which the infected person has traveled). Malaria cases that have been confirmed by blood smear are identified by health-care providers and/or laboratories. Each slide-confirmed case is reported to local and/or state health departments and to CDC through NMSS on a uniform case report form that contains clinical, laboratory, and epidemiologic information. CDC staff review all report forms at the time of receipt and request additional information if necessary (e.g., when no recent travel to a malaria-endemic country is reported). Other cases are recorded when health-care providers directly phone CDC to request assistance with the diagnosis and treatment of malaria. All cases that have been acquired in the United States are fully investigated, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case report forms is entered into a computer data base and analyzed annually. Definition of Terms The following definitions are used in this report:
This report also uses terminology derived from the recommendations of the World Health Organization (4). Definitions of the following terms are included for reference:
Microscopic Diagnosis of Malaria The early diagnosis of malaria requires that physicians consider malaria in the differential diagnosis of illness in every patient who has fever; the evaluation of such a patient should include taking a comprehensive travel history. If malaria is suspected, a Giemsa-stained smear of the patient's peripheral blood should be examined for parasites. Thick and thin blood smears must be prepared properly because the accuracy of diagnosis depends on the quality of the blood film and the experience of the laboratory personnel. * (See Appendix A for proper procedures necessary for accurately diagnosing malaria.) RESULTS General Surveillance CDC received reports of 1,014 malaria cases that had onset of symptoms during 1994 among persons in the United States and its territories, representing a 20% decline from the 1,275 cases reported for 1993 (5). In 1994, five of the 1,014 persons acquired malaria while in the United States compared with 11 of 1,275 persons in 1993. Since 1973, malaria in civilians has accounted for most cases reported to CDC (Table_1). During 1994, 524 cases occurred in U.S. civilians compared with 519 cases reported for 1993 (Figure_1). The number of cases in foreign civilians decreased by 18%, from 453 cases in 1993 to 370 cases in 1994. Among U.S. military personnel, the 38 cases reported in 1994 constituted an 86% decrease from the 278 cases in 1993. Eighty-four percent of the cases among U.S. military personnel in 1993 were acquired during deployment in Somalia (5). Among military personnel, the number of cases reported in 1994 (i.e., 38) was similar to the number of cases reported in the years before the large-scale deployment in Somalia. Plasmodium Species The infecting species of Plasmodium was identified in 964 (95%) of the 1,014 cases in 1994 (Table_2). P. vivax and P. falciparum were identified in blood smears from 43.5% and 43.6% of infected persons, respectively. The 441 P. vivax cases reported in 1994 represented a 33% decrease from the 663 cases in 1993. The 442 P. falciparum cases reported in 1994 represented a 3% decrease from the 457 cases in 1993. Area of Acquisition and of Diagnosis Most of the imported malaria cases had been acquired in Africa (517 {51%} cases) and Asia (253 {25%} cases) (Table_3). In comparison with 1993, 234 fewer reported cases were acquired in Africa during 1994; this decline primarily reflected the decrease in the number of cases acquired by military personnel in Somalia. The number of cases acquired in Asia remained stable from 1993 (259 cases) to 1994 (253 cases). In the United States, cases are reported by the state in which they are diagnosed (Figure_2). Seventy-four cases were reported from New York State (excluding New York City) in 1994, representing a 61% decrease from the 191 cases in 1993. In 1993, 107 of the cases reported from New York State occurred in military personnel returning from Somalia. New York City, which began reporting cases to CDC in 1993, reported 107 cases for 1994, compared with 130 in 1993. Interval Between Arrival and Onset of Illness The infecting Plasmodium species and the interval between arrival in the United States and the onset of illness were known for 748 (74%) persons who had malaria. Symptoms began after arrival in the United States for 687 (92%) of these persons. Clinical malaria developed within 29 days after arrival in 284 (90%) of the 314 P. falciparum cases and in 83 (26%) of the 319 P. vivax cases (Table_4). Only 25 (4%) of the 687 persons became ill greater than 1 year after arriving in the United States. An additional 61 persons reported becoming ill before arriving in the United States. Symptoms developed within 1 week before arrival in 37 (61%) of these persons; the remaining 24 persons reported that symptoms began 8-89 days before arrival. Imported Malaria Cases Imported Malaria in Military Personnel Thirty-eight cases of imported malaria in U.S. military personnel were reported for 1994. Seventeen of these cases occurred in personnel of the U.S. Army; seven cases, the U.S. Marine Corps; eight cases, the U.S. Air Force; and four cases, the U.S. Navy. Two cases occurred in military personnel for whom the service branch was not identified. Imported Malaria in Civilians A total of 889 imported malaria cases occurred in civilians (Table_5). Of these, 519 (58%) cases occurred in U.S. residents, and 370 (42%) occurred in residents of other countries. Of the 519 U.S. civilians who had imported malaria, 303 (58%) had traveled to Africa, representing a 10% increase over the 276 cases acquired in this region during 1993. Of the imported cases that occurred in U.S. civilians in 1994, 102 (20%) had been acquired in Asia. Of the 370 imported cases among foreign civilians, 173 (47%) had been acquired in Africa and 128 (35%) in Asia. Use of Antimalarial Chemoprophylaxis Information concerning use of chemoprophylaxis was known for 487 (94%) of the 519 U.S. civilians who had imported malaria. Of these 487 persons, 257 (53%) had not taken chemoprophylaxis and 105 (22%) had not taken a drug recommended by CDC for the area visited. Eighty-six (18%) persons had taken a medication recommended by CDC (6). Of these persons, 69 had taken mefloquine weekly; three had taken doxycycline daily; and 14, who had traveled only in areas where chloroquine-resistant malaria has not been documented, had taken chloroquine weekly. Of the 86 malaria cases that developed in persons who had taken a recommended antimalarial drug, 44 (51%) were caused by P. vivax or P. ovale and occurred greater than or equal to 45 days after the person arrived in the United States. These cases were consistent with relapsing infections and, thus, did not indicate failures of prophylaxis. Information was insufficient to assess whether six cases of P. vivax malaria were relapsing infections. Only four cases of P. vivax malaria occurred within 45 days after the person returned to the United States. The remaining 32 malaria cases that occurred in persons who had taken a recommended antimalarial drug for chemoprophylaxis included 24 cases of P. falciparum, two cases of P. malariae, and six cases in which the infecting species was not identified. The two cases of P. malariae both occurred greater than 45 days after the person returned to the United States and may represent late recrudescence of P. malariae infection. Of the 86 persons who reported having taken a recommended antimalarial drug for chemoprophylaxis, information concerning the dosing regimen was available for 20 (23%). Thirteen (65%) of these 20 persons reported that they had taken incorrect or irregular doses of the recommended medications. Only seven (35%) of those who had taken a recommended drug for the area in which they had traveled also reported correctly following the dosing regimen. The purpose of travel to malaria-endemic areas was reported for 276 (53%) of the 519 U.S. civilians who had imported malaria (Table_6). Of these, the largest percentage (12%) had traveled to visit a friend or relative. Malaria Acquired in the United States Congenital Malaria No cases of congenital malaria were reported for 1994. Cryptic Malaria Three cases of cryptic malaria were reported for 1994. All three persons acquired P. vivax infection in Houston, Texas, through presumed mosquitoborne transmission (7). Case 1. On July 8, 1994, a 62-year-old man was hospitalized in Houston because of an 8-day history of fever, chills, increased sweating, and vomiting. His temperature at the time of admission was 104.0 F (40.0 C). On July 11, P. vivax parasites were identified on a peripheral blood smear. The man did not have a history of blood transfusions, intravenous-drug use, tattoos, or previous malarial infection. He had not traveled to a malaria-endemic area since 1956. He recovered after treatment with chloroquine and primaquine. Case 2. On July 8, 1994, a 37-year-old man was admitted to a different hospital in Houston because of fever, chills, increased sweating, headache, shortness of breath, nausea, and vomiting of 3 weeks' duration. He reported no travel outside of the United States, blood transfusions, tattoos, or previous malarial infection. He had not used intravenous drugs for at least 8 years before this illness. He slept in a shack without screens behind a relative's house. At the time of admission to the hospital, he had a fever of 102.8 F (39.3 C). P. vivax parasites were identified on a routine peripheral blood smear. The man recovered after initial treatment with chloroquine; he received primaquine during the investigation in August to prevent possible relapse. Case 3. On December 4, 1994, a 50-year-old man was hospitalized in Houston because of a 2-week history of altered mental status, fever, and headache; his temperature at the time of hospital admission was 100.0 F (37.8 C). An examination of a peripheral blood smear demonstrated the presence of P. vivax parasites. The man recovered after treatment with chloroquine and primaquine. He had been hospitalized twice at two different hospitals in August 1994 because of similar symptoms that had begun during July and early August. During the second admission, his illness was diagnosed as viral meningitis; one thick blood smear obtained on August 23 was reported as being negative for malarial parasites, and acute- and convalescent-phase immunoglobulin M enzyme-linked immunosorbent assay titers for St. Louis encephalitis were negative. The blood smear obtained on August 23 was unavailable for review by CDC; however, serum samples from the hospitalizations in August and December were tested for malarial antibody by an indirect immunofluorescent assay. The serologic test was positive for P. vivax (titers of 1:64, 1:256, and 1:256 on August 23, August 30, and December 6, respectively). These results suggested that the P. vivax infection occurred before December and that the episode in December was likely a relapse, which can only result from mosquitoborne transmission and not from person-to-person transmission via blood products. Because the man had not traveled to a malaria-endemic country, local mosquitoborne transmission was likely the cause of the initial infection. These three cases were linked geographically and temporally. All three persons resided within a 3-mile radius of one another and first became ill in July. They were not acquainted and had had no previous contact with one another. All three had prolonged nighttime exposures to mosquitoes. On August 4, adult Anopheles quadrimaculatus mosquitoes (i.e., competent potential vectors of malaria) were trapped near the residences of the patients described in cases 1 and 2, although no active breeding sites were identified at that time. Induced Malaria Two cases of transfusion-induced malaria were reported for 1994. Both cases occurred in Texas. Case 1. Between March 24 and April 1, 1994, a 59-year-old woman who lived in Houston received 8 units of packed red blood cells. On April 20, she became ill with fever and disseminated intravascular coagulation; an examination of a peripheral blood smear demonstrated the presence of P. falciparum parasites (7% of red blood cells were infected). The diagnosis was confirmed microscopically at CDC. She did not have a history of travel to malaria-endemic areas, and she reported no previous malarial infection. She was treated with quinine and doxycycline and recovered without complications. Serum specimens were obtained from seven of eight donors of the blood that the woman had received in early March and April. One donor could not be located. Serum specimens from six donors were negative for antimalarial antibodies. Serum from the remaining donor was positive for P. falciparum antibodies (1:4,096). When this donor was questioned, he reported having had a malarial infection in 1991 and having resided in Nigeria during 1988-1992. The donor had no symptoms of acute malaria at the time of the interview, and no parasites were detected on his peripheral blood smear. He was treated with quinine and tetracycline. Case 2. On October 23, 1994, a 46-year-old man underwent cardiac surgery at a hospital in Houston. During his hospitalization, he received 7 units of packed red blood cells and 2 units of fresh frozen plasma. On November 9, he was readmitted because of a 6-day history of fever and a surgical wound infection. On November 16, an examination of a peripheral blood smear demonstrated the presence of P. falciparum parasites (24% of red blood cells were infected); this diagnosis was confirmed by CDC. The man had not traveled to a malaria-endemic area. He was treated initially with chloroquine, which was changed to quinine and doxycycline after P. falciparum infection was confirmed. Serum samples were obtained from six of the seven persons who had donated the packed red blood cells. One donor could not be located. Serum samples from five donors were negative for antimalarial antibodies by immunofluorescent antibody assay. Serum from the remaining donor was positive for antibodies to P. falciparum (1:4,096). This donor had lived in Ghana for several years and had returned to the United States in 1992. An examination of the donor's peripheral blood smear at the time of the investigation did not demonstrate the presence of malarial parasites. He was treated with quinine and sulfadoxine-pyrimethamine. Deaths attributed to Malaria During 1994, four deaths were attributed to malaria. Case 1. A 47-year-old man became ill 1 week after returning to his residence in Florida following a 3-week visit to Haiti, where he had been born. He was evaluated at a hospital emergency department because of a 3-day history of fever and lower back pain, at which time his illness was diagnosed as a viral illness. He had not taken antimalarial chemoprophylaxis during his trip. He was treated with amoxicillin, acetominophen, and codeine and was not hospitalized. He returned to the hospital 24 hours later because of recurrent fever and chills and unabated back pain, and he was admitted because of a suspected dissecting aortic aneurysm. An examination of a peripheral blood smear at the time of admission demonstrated the presence of P. falciparum parasites. He was treated with intravenous quinidine gluconate, and then oral quinine sulfate combined with oral doxycycline. On November 30, he had hypotension, acute renal failure, and disseminated intravascular coagulation. He died on December 1. Case 2. On October 4, 1994, a 59-year-old man who had been born in Asia and who resided in New York returned from India. He had not taken antimalarial chemoprophylaxis during his trip. He had a past history of aortic and mitral valve replacement. On October 6, he was admitted to a hospital in New York because of a 9-day history of fever, chills, jaundice, and myalgia; his illness was diagnosed as bacterial endocarditis and severe respiratory failure requiring mechanical ventilation. An examination of the man's blood smear at the time of admission demonstrated the presence of P. falciparum, and treatment with intravenous quinidine gluconate and doxycycline was initiated. During the next 2 days, cerebral malaria and renal failure developed in the man. On October 11, he became comatose after a cardiac arrest. He died on October 19. A postmortem examination demonstrated evidence of anoxic encephalopathy, acute renal failure, and prosthetic aortic and mitral valve endocarditis. The cause of death was determined to be anoxic encephalopathy secondary to cerebral malaria. Case 3. On June 7, 1994, a 27-year-old man was hospitalized in San Juan, Puerto Rico, because of a 1-week history of fever, chills, vomiting, and diarrhea. He had arrived in Puerto Rico from the Dominican Republic 2 weeks earlier. A physical examination of the man at the time of admission indicated only mild jaundice. His hemoglobin was 9.3 g/dL; white blood cell count, 8,900; platelet count, 16,000; and bilirubin, 2.8 mg/dL. The illness was diagnosed initially as bacterial gastroenteritis, and he was treated with antibiotics. On June 9, he had renal failure, and an examination of his peripheral blood films demonstrated the presence of P. falciparum parasitemia (greater than 50% of erythrocytes were infected). His hemoglobin had decreased to 7.1 g/dL. Antimalarial therapy with oral quinine and doxycycline was initiated. Respiratory acidosis developed in the man, and he died the next day. Case 4. During November 1993, a 74-year-old man returned to his residence in California after a trip to India. He had been born in Asia, and he traveled frequently between India and his residence. He had not taken antimalarial prophylaxis during his most recent trip, despite a previous history of malaria in India in June 1993. On January 27, 1994, he went to a local emergency department because of a 10-12 day history of dyspnea, chest pain, and recurrent fever and chills. At the time of hospital admission, an examination of the man indicated evidence of a recent myocardial infarction and ischemic cardiomyopathy. An examination of his peripheral blood smears demonstrated the presence of P. vivax infection, and he was treated with chloroquine and primaquine. On the second day of hospitalization, respiratory distress requiring mechanical ventilation developed. On February 3, no malarial parasites were seen on thick or thin blood smears. That day, the man had cardiogenic shock and died 24 hours later. No autopsy was performed. Although the P. vivax infection may have contributed to the complicated clinical course of the man's illness, it is unlikely that malaria was the immediate cause of death. Unlike P. falciparum infection, P. vivax infection is seldom fatal, and myocardial dysfunction is not a recognized complication of parasitemia. DISCUSSION A total of 1,014 cases of malaria were reported to NMSS for 1994, representing a 20% decrease from the 1,275 cases reported for 1993. The 240 fewer cases in U.S. military personnel during 1994 (i.e., after the deployment of troops in Somalia was ended in 1993) accounted for 93% of this decrease. Although the number of cases occurring in U.S. civilians was unchanged from the 1993 level, the number of cases among foreign civilians decreased by 18%. While the percentage of cases caused by P. vivax infection decreased from 52% in 1993 to 44% in 1994, the percentage caused by P. falciparum increased from 36% to 44%. Most of the cases acquired in Somalia in 1993 resulted from infection with P. vivax. Of those U.S. civilians for whom antimalarial chemoprophylaxis information was available, only 86 (18%) infections occurred in persons who reported having taken a recommended chemoprophylactic agent while traveling abroad. An earlier review of deaths attributed to malaria in the United States identified several risk factors for fatal malaria, including failure to take recommended antimalarial chemoprophylaxis, refusal or delay in seeking medical care, and misdiagnosis (8). A combination of these factors contributed to the four deaths reported for 1994. Treatment for malaria should be initiated immediately after the diagnosis has been confirmed by a positive blood smear. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, the parasite density, and the patient's clinical status (9). Although non-falciparum malaria rarely causes severe illness, persons diagnosed as having P. falciparum infection are at risk for severe, life-threatening complications. Health-care workers are encouraged to consult appropriate sources for malaria treatment recommendations or call CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, at (770) 488-7760. Detailed recommendations for preventing malaria are available 24 hours a day from the CDC International Travelers Hotline, which can be accessed by telephone or facsimile ({888} 232-3228) or CDC's World-Wide Web server (http://www.cdc.gov/). In addition, CDC annually publishes updated recommendations in the Health Information for International Travel (6), which is available through the Superintendent of Documents, U.S. Government Printing Office, Washington, DC 20402-9235; telephone (202) 512-1800. References
* To confirm the diagnosis of questionable cases and to obtain appropriate treatment recommendations, contact either the state or local health department or CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, Malaria Section; telephone (770) 488-7760. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. TABLE 1. Number of malaria cases* in U.S. and foreign civilians and U.S. military personnel -- United States, 1966-1994 ========================================================================================= Year U.S. military personnel U.S. civilians Foreign civilians Unknown Total ----------------------------------------------------------------------------------------- 1966 621 89 32 22 764 1967 2,699 92 51 15 2,857 1968 2,567 82 49 0 2,698 1969 3,914 90 47 11 4,062 1970 4,096 90 44 17 4,247 1971 2,975 79 69 57 3,180 1972 454 106 54 0 614 1973 41 103 78 0 222 1974 21 158 144 0 323 1975 17 199 232 0 448 1976 5 178 227 5 415 1977 11 233 237 0 481 1978 31 270 315 0 616 1979 11 229 634 3 877 1980 26 303 1,534 1 1,864 1981 21 273 809 0 1,103 1982 8 348 574 0 930 1983 10 325 468 0 803 1984 24 360 632 0 1,016 1985 31 446 568 0 1,045 1986 35 410 646 0 1,091 1987 23 421 488 0 932 1988 33 550 440 0 1,023 1989 35 591 476 0 1,102 1990 36 558 504 0 1,098 1991 22 585 439 0 1,046 1992 29 394 481 6 910 1993 278 519 453 25 1,275 1994 38 524 370 82 1,014 ----------------------------------------------------------------------------------------- * An episode of microscopically confirmed malaria parasitemia in any person (symptomatic or asymptomatic) diagnosed in the United States, regardless of whether the person experienced previous episodes of malaria while outside the country. A subsequent attack of malaria occurring in a person is counted as an additional case if the demonstrated Plasmodium species differs from the initially identified species. A subsequent attack of malaria occurring in a person while in the United States could indicate a relapsing infection or treatment failure resulting from drug resistance if the demonstrated Plasmodium species is the same species identified previously. ========================================================================================= Return to top. Figure_1 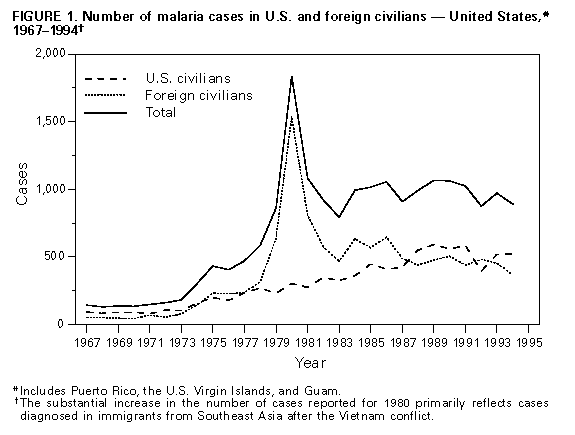 "> ">Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Number of malaria cases, by Plasmodium species --
United States, 1993 and 1994
===========================================================
1993 1994
---------------- -----------------
Plasmodium species No. (%) No. (%)
-----------------------------------------------------------
P. vivax 663 ( 52.0) 441 ( 43.5)
P. falciparum 457 ( 35.8) 442 ( 43.6)
P. malariae 53 ( 4.2) 43 ( 4.2)
P. ovale 41 ( 3.2) 34 ( 3.4)
Undetermined 59 ( 4.6) 50 ( 4.9)
Mixed 2 ( 0.2) 4 ( 0.4)
Total 1,275 (100.0) 1,014 (100.0)
===========================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3. Number of malaria cases, by Plasmodium species and area of acquisition -- United States, 1994 --
==========================================================================================================================
Plasmodium species
----------------------------------------------------------------------------
Area of acquisition P. vivax P. falciparum P. malariae P. ovale Mixed Unknown Total
--------------------------------------------------------------------------------------------------------------------------
AFRICA 40 380 32 33 1 31 517
Algeria 0 1 0 0 0 0 1
Angola 0 3 0 0 0 0 3
Benin 1 1 0 0 0 0 2
Botswana 0 1 0 0 0 0 1
Burkina Faso 0 2 0 0 0 0 2
Cameroon 0 2 2 2 0 0 6
Central African Republic 0 2 0 0 0 0 2
Chad 0 4 0 0 0 0 4
Congo 0 2 0 1 0 0 3
Djibouti 0 0 0 0 0 0 0
Egypt 1 0 0 0 0 0 1
Equatorial Guinea 0 0 0 0 0 0 0
Ethiopia 4 0 0 0 0 0 4
Gabon 0 1 0 0 0 1 2
Gambia 0 10 0 0 0 0 10
Ghana 2 47 5 3 0 4 61
Guinea 0 4 0 0 0 1 5
Guinea-Bissau 0 0 0 0 0 0 0
Ivory Coast 0 14 1 2 0 4 21
Kenya 5 16 1 6 0 4 32
Liberia 1 11 3 1 0 4 20
Madagascar 3 0 0 0 0 0 3
Malawi 0 2 0 0 0 0 2
Mali 0 5 0 0 0 0 5
Mauritania 0 0 0 0 0 0 0
Mozambique 0 1 0 0 0 0 1
Niger 0 0 0 0 0 0 0
Nigeria 2 136 12 6 0 9 165
Rwanda 0 0 0 0 1 0 1
Senegal 0 5 2 0 0 0 7
Sierra Leone 2 10 0 1 0 0 13
Somalia 6 1 1 0 0 0 8
South Africa 0 3 0 0 0 0 3
Sudan 6 6 0 0 0 1 13
Tanzania 0 2 0 0 0 1 3
Togo 0 3 0 0 0 0 3
Uganda 0 7 0 3 0 0 10
Zaire 1 9 0 0 0 0 10
Zambia 1 3 0 0 0 0 4
Zimbabwe 0 1 0 0 0 0 1
Africa,Central* 0 4 0 0 0 0 4
Africa,East* 3 16 1 3 0 0 23
Africa,South* 0 0 0 1 0 0 1
Africa,West* 0 16 0 1 0 1 18
Africa,Unspecified* 2 29 4 3 0 1 39
ASIA 204 30 6 0 1 12 253
Afghanistan 0 0 0 0 0 0 0
Bangladesh 1 0 0 0 0 0 1
Cambodia 0 0 0 0 0 0 0
China 0 0 0 0 0 0 0
India 153 23 4 0 1 8 189
Indonesia 8 0 1 0 0 1 10
Laos 2 2 0 0 0 0 4
ASIA (cont'd)
Malaysia 0 0 0 0 0 0 0
Myanmar (Burma) 1 0 0 0 0 1 2
Nepal 0 0 0 0 0 0 0
Pakistan 17 3 0 0 0 0 20
Philippines 2 0 0 0 0 0 2
Saudi Arabia 0 0 0 0 0 0 0
Sri Lanka 1 1 1 0 0 0 3
Thailand 0 0 0 0 0 0 0
Vietnam 10 1 0 0 0 1 12
Yemen 0 0 0 0 0 0 0
Asia,Southeast* 3 0 0 0 0 0 3
Asia,Unspecified* 6 0 0 0 0 1 7
Middle East,Unspecified* 0 0 0 0 0 0 0
CENTRAL AMERICA AND CARIBBEAN 119 21 3 0 1 2 146
Belize 7 0 1 0 0 0 8
Caribbean,Unspecified* 0 0 0 0 0 0 0
Costa Rica 4 0 1 0 0 0 5
Cuba 0 1 0 0 0 0 1
Dominican Republic 0 1 0 0 0 0 1
El Salvador 4 0 0 0 0 0 4
Guatemala 19 2 1 0 0 0 22
Haiti 0 9 0 0 0 0 9
Honduras 55 6 0 0 1 1 63
Nicaragua 15 2 0 0 0 0 17
Panama 0 0 0 0 0 1 1
Central America,Unspecified* 15 0 0 0 0 0 15
NORTH AMERICA 14 2 0 0 0 2 18
Canada 0 0 0 0 0 0 0
Mexico 11 0 0 0 0 2 13
United States 3 2 0 0 0 0 5
SOUTH AMERICA 13 0 1 0 1 0 15
Brazil 8 0 0 0 0 0 8
Colombia 1 0 0 0 0 0 1
Ecuador 0 0 0 0 0 0 0
French Guiana 0 0 0 0 0 0 0
Guyana 0 0 0 0 1 0 1
Peru 2 0 0 0 0 0 2
Venezuela 1 0 0 0 0 0 1
South America,Unspecified* 1 0 1 0 0 0 2
OCEANIA 25 1 0 1 0 0 27
Papua New Guinea 17 1 0 1 0 0 19
Solomon Islands 8 0 0 0 0 0 8
Vanuatu 0 0 0 0 0 0 0
Oceania,Unspecified* 0 0 0 0 0 0 0
Unknown* 26 8 1 0 0 3 38
Total 441 442 43 34 4 50 1,014
--------------------------------------------------------------------------------------------------------------------------
* Country unspecified.
==========================================================================================================================
Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 4. Number of imported malaria cases, by Plasmodium species and by interval between date of arrival in the country and onset of illness --
United States, 1994
====================================================================================================================================================
Plasmodium species
------------------------------------------------------------------------------------------------
P. vivax P. falciparum P. malariae P. ovale Total
----------------------- ---------------------- ---------------------- ------------------- -----------------
Interval (days) No. (%) No. (%) No. (%) No. (%) No. (%)
----------------------------------------------------------------------------------------------------------------------------------------------------
0- 29 83 ( 26.0) 284 ( 90.4) 20 ( 64.5) 4 ( 17.4) 391 ( 56.9)
30- 89 67 ( 21.0) 21 ( 6.7) 4 ( 12.9) 7 ( 30.4) 99 ( 14.4)
90-179 75 ( 23.5) 6 ( 1.9) 5 ( 16.1) 6 ( 26.1) 92 ( 13.4)
180-364 74 ( 23.2) 2 ( 0.6) 1 ( 3.2) 3 ( 13.0) 80 ( 11.6)
>=365 20 ( 6.3) 1 ( 0.3) 1 ( 3.2) 3 ( 13.0) 25 ( 3.6)
Total 319 (100.0) 314 (100.0) 31 (100.0) 23 (100.0) 687 (100.0)
====================================================================================================================================================
Return to top. Figure_2 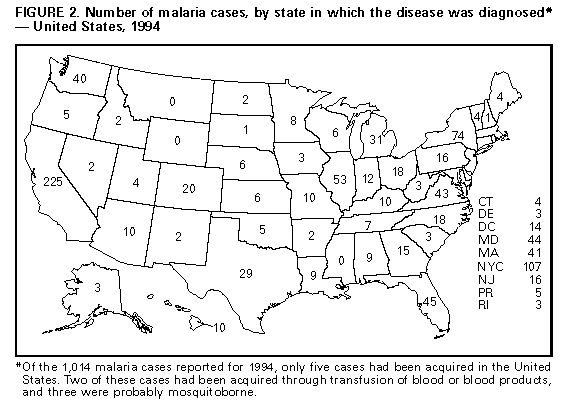 "> ">Return to top. Table_5 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 5. Number of imported malaria cases in U.S. and foreign civilians, by area of acquisition
-- United States, 1994
=====================================================================================================
U.S. civilians Foreign civilians Total
---------------- ----------------- --------------------
Area of acquisition No. (%) No. (%) No. (%)
-----------------------------------------------------------------------------------------------------
Africa 303 ( 58.4) 173 ( 46.8) 476 ( 53.5)
Asia 102 ( 19.7) 128 ( 34.6) 230 ( 25.9)
Caribbean 5 ( 1.0) 4 ( 1.1) 9 ( 1.0)
Central America 62 ( 11.9) 43 ( 11.6) 105 ( 11.9)
Mexico 7 ( 1.3) 5 ( 1.4) 12 ( 1.3)
Oceania 24 ( 4.6) 3 ( 0.8) 27 ( 3.1)
South America 9 ( 1.7) 3 ( 0.8) 12 ( 1.3)
Unknown 7 ( 1.3) 11 ( 3.0) 18 ( 2.0)
Total 519 (100.0) 370 (100.0) 889 (100.0)
=====================================================================================================
Return to top. Table_6 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 6. Number of imported malaria cases in U.S. civilians, by
purpose of travel at the time of acquisition -- United States, 1994
===================================================================
Imported cases
---------------------------
Category No. (%)
-------------------------------------------------------------------
Business representative 35 ( 6.7)
Government employee 4 ( 0.8)
Missionary 48 ( 9.2)
Peace Corps worker 5 ( 1.0)
Refugee 3 ( 0.6)
Sailor 5 ( 1.0)
Teacher/Student 50 ( 9.6)
Tourist 52 ( 10.0)
Visitor of a friend or relative 60 ( 11.6)
Other 14 ( 2.7)
Unknown 243 ( 46.8)
Total 519 (100.0)
===================================================================
Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|