 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Adoption of Hospital Policies for Prevention of Perinatal Group B Streptococcal Disease -- United States, 1997Group B streptococcal (GBS) infections are the leading bacterial cause of disease and deaths among newborns in the United States. In 1993, the annual cost of caring for newborns with sepsis caused by group B Streptococcus was an estimated $294 million (1). A survey of hospital GBS disease prevention practices in 1994 indicated that those hospitals with a prenatal screening policy had fewer neonatal GBS disease cases (2). In 1996, to promote a coordinated approach to prevention, CDC issued consensus guidelines about GBS disease prevention that were endorsed by the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics (3-5). These consensus guidelines recommend using either a screening-based strategy or a risk-based strategy for identifying women who should receive intrapartum antimicrobial prophylaxis. To evaluate adoption of the consensus guidelines, in 1997, hospitals in eight surveillance areas were surveyed, and the results were compared with findings of a similar survey conducted in 1994. The proportion of hospitals with prevention policies in each site was compared with the site's rate of early-onset disease to assess the impact of the prevention policies. This report presents the survey findings, which indicate that more hospitals have adopted GBS disease prevention policies since issuance of the consensus guidelines. In 1997, a comprehensive survey was mailed to all 189 hospitals providing obstetric services in the following surveillance areas: the 20-county metropolitan statistical area of Atlanta, Georgia (n=31 hospitals); the three-county San Francisco Bay area of California (n=24); the seven-county Rochester area of New York (n=12); the seven-county Twin Cities area of Minnesota (n=20); the three-county Portland area of Oregon (n=13); five urban counties in Tennessee (n=24); and the entire states of Connecticut (n=30) and Maryland (n=35). The survey assessed laboratory methods used to identify group B streptococci, several patient demographic characteristics, and obstetric policies. In 1994, three separate surveys evaluating the same topics were sent to all 295 hospitals providing obstetric services in an eight-county Atlanta area of Georgia (n=20); the three-county San Francisco Bay area of California (n=29); and the entire states of Maryland (n=36), Missouri (n=123), and Oklahoma (n=87) (2). Survey responses to the 1997 study were compared with those to the 1994 study using the chi-square test. For the hospitals that responded to the survey in both years, survey responses were compared using McNemar's test. To identify GBS disease cases, surveillance personnel regularly requested standardized reports of cases of invasive GBS disease from each laboratory that served acute-care hospitals within the specified surveillance areas. Periodic audits of laboratory records were conducted to validate completeness of reporting. A case of early-onset neonatal GBS disease was defined as isolation of group B streptococci from a normally sterile site (e.g., blood or cerebrospinal fluid) from a resident of an area under surveillance with illness onset before 7 days of age. To calculate the incidence of early-onset GBS disease for the surveillance areas, the number of live-born infants for 1996 was obtained from the respective state health departments or from CDC's National Center for Health Statistics. The incidence of GBS disease in 1996 was not available for the seven counties in New York. Of the 189 hospitals surveyed in 1997, a total of 177 (94%) completed the survey. Of those that responded, 103 (58%) had a general GBS disease prevention policy, and 82 (46%) had a written policy. To determine who should receive intrapartum antibiotics, 50 (28%) followed the screening-based strategy in the consensus guidelines, 36 (20%) followed the risk-based strategy in the consensus guidelines, and seven (4%) followed both strategies. Most of these policies were established in 1996 and 1997 (Figure_1). Hospitals with a neonatal intensive-care unit (NICU) were equally likely as those without an NICU to have a GBS disease prevention policy (54 {59%} of 91 versus 49 {57%} of 86). Although more hospitals with academic affiliations had GBS disease prevention policies than hospitals without those affiliations (38 {67%} of 57 versus 64 {54%} of 118), the difference was not statistically significant. Of the 295 hospitals surveyed in 1994, a total of 154 (52%) completed the obstetric policy section, 234 (79%) completed the hospital patient demographics section, and 247 (84%) completed the laboratory section (2). Compared with 1994, a higher proportion of hospitals in 1997 had obstetric policies for preventing neonatal GBS disease and recommended appropriate laboratory methods (including the use of selective broth media) for isolation of group B streptococci (Table_1). In 1997, 80% of GBS disease prevention policies were written policies, compared with 34% in 1994. Of those hospitals with prenatal screening policies, the proportion with policies consistent with the 1996 recommendation to screen all women and to culture specimens from both the vagina and rectum increased from 1994 to 1997. Among the hospitals for which a response was available for both 1994 and 1997, the proportion of hospitals with written policies and recommendations for using appropriate selective broth culture media increased from 1994 to 1997 (Table_1). According to 1996 surveillance data from seven areas, the incidence of early-onset GBS disease ranged from 0.6 cases per 1000 live births to 1.8 cases per 1000 live births. Geographic areas in which a higher proportion of hospitals had GBS disease prevention policies had lower incidences of early-onset GBS disease (R2=0.62; p=0.03) (Figure_2). Reported by: P Daily, MPH, L Gelling, MPH, N Mukerjee, MPH, G Rothrock, MPH, A Reingold, MD, Emerging Infections Program, San Francisco; D Vugia, MD, S Waterman, MD, State Epidemiologist, California State Dept of Health Svcs. C Morin, MPH, Q Phan, N Barrett, MS, P Mshar, JL Hadler, MD, State Epidemiologist, Connecticut Dept of Public Health. W Baughman, MSPH, M Farley, MD, D Stephens, MD, Veterans Administration Medical Svcs and Emory Univ School of Medicine, Atlanta; P Blake, MD, K Toomey, MD, State Epidemiologist, Div of Public Health, Georgia Dept of Human Resources. L Billmann, MPH, L Harrison, MD, Johns Hopkins Univ, Baltimore; DM Dwyer, MD, State Epidemiologist, Maryland State Dept of Health and Mental Hygiene. J Rainbow, MPH, M Osterholm, PhD, State Epidemiologist, Minnesota Dept of Health. B Hathaway, D Morse, MD, P Smith, MD, State Epidemiologist, New York State Dept of Health. K Stefonek, MPH, D Fleming, MD, State Epidemiologist, State Health Div, Oregon Dept of Human Resources. B Barnes, MS, L Lefkowitz, MD, Dept of Preventive Medicine, Vanderbilt Medical Center, Nashville, Tennessee. ABCs and the Emerging Infections Program network, Respiratory Diseases Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases; and an EIS officers, CDC. Editorial NoteEditorial Note: Early-onset GBS disease can be prevented by targeted use of antimicrobial prophylaxis after onset of labor or rupture of membranes (3). The preventable portion of early-onset GBS disease has been estimated as 78% for the screening-based approach and as 41% for the risk-based approach (6). Surveillance data for 1993-1995, which included four of the eight sites surveyed in 1997, revealed a significant decrease in the incidence of early-onset GBS disease (7). Since the consensus guidelines were issued in 1996, substantially more hospitals have adopted GBS disease prevention policies and laboratory methods for isolating group B Streptococcus that may be effective against early-onset GBS disease. This increase in adoption of GBS disease prevention policies and appropriate laboratory methods should result in a continued decrease in the incidence of early-onset GBS disease. The findings of the analysis that combined 1996 GBS surveillance data with hospital survey results indicate that geographic areas in which more hospitals have GBS disease prevention policies have less early-onset GBS disease. Although the survey measured policies and not actual practices, this finding suggests that prevention policies are being followed. Ongoing studies in the surveillance areas will assess how strongly GBS disease prevention policies affect hospital-specific rates of early-onset disease. In 1994, hospitals with academic affiliations were more likely to have prevention policies than those without academic affiliations (36% versus 18%; p=0.03), and hospitals with an NICU also were more likely than those without an NICU to have prevention policies (53% versus 31%; p=0.02) (2). However, by 1997, differences between these specialized hospitals and others were no longer statistically significant. These data suggest that issuance of the consensus guidelines has resulted in increased use of GBS disease prevention strategies by providers in community hospitals. The findings in this report of an increase in the number of hospitals with GBS disease prevention policies suggest that issuance of consensus guidelines may have played a role in adoption of such policies. However, nearly half of the hospitals surveyed are not using either of the two strategies recommended in the consensus guidelines. In addition, approximately half of the hospitals are not using appropriate selective broth culture media. The use of selective broth media is key to an effective prenatal screening program, improving the yield of prenatal screening cultures by up to 50% (8). Strategies to prevent neonatal GBS disease should include efforts directed toward health-care providers in both community and academic institutions. These efforts should focus on increasing awareness of GBS disease as a preventable disease, increasing use of the screening-based strategy or the risk-based strategy, and increasing use of selective broth media. Integration of GBS disease prevention efforts with programs for prevention of other perinatal diseases (e.g., maternal use of folic acid to prevent neural tube defects {9} and screening and vaccination to prevent perinatal hepatitis B infections {10}) may increase awareness and acceptance of these perinatal disease prevention strategies. Tracking the progress of strategies for preventing these diseases should continue to be an important national health objective. Copies of the GBS disease prevention guidelines, clinical posters, technical slide sets for health professionals, and both printed and video material for prenatal patients are available from CDC's Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, Mailstop C-23, 1600 Clifton Road, N.E., Atlanta, GA 30333, or from the World-Wide Web at http://www.cdc.gov/ncidod/dbmd/gbs/. References
Figure_1 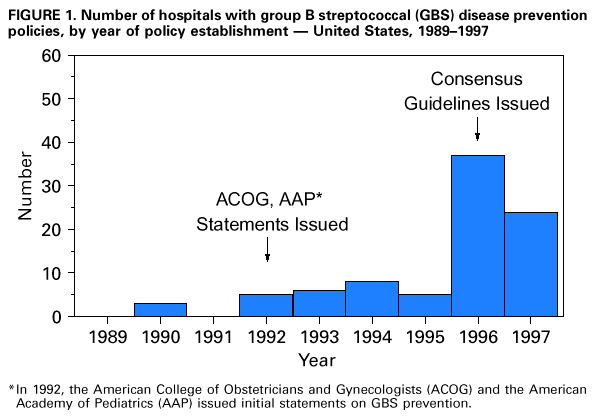 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Total respondents and number and percentage of hospitals with group B
streptococcal (GBS) disease prevention policies, by policies and policy components --
United States, 1994 and 1997
=================================================================================================================
1994 1997
------------------------- -------------------------
No. No.
Total with Total with
Policies and policy contents respondents policy (%) respondents policy (%) p value
---------------------------------------------------------------------------------------------------------------
Respondents to obstetric policy
component of survey
Any GBS disease prevention
policy 147 58 (39%) 177 103 (58%) <0.01
Written policy 147 20 (14%) 177 82 (46%) <0.01
Intrapartum antibiotic prophylaxis
policy 147 50 (35%) 177 95 (54%) <0.01
Indication for treatment
Positive for group B
Streptococcus 45 * 16 (36%) 95 50 (53%) 0.09
Risk factors identified by
guidelines + 45 8 (18%) 95 36 (38%) 0.03
Both of the above 45 13 (29%) 95 7 ( 7%) <0.01
Prenatal screening policy 145 36 (25%) 177 66 (37%) 0.02
Indication for screening
Screen all women & 32 * 9 (26%) 66 34 (52%) 0.05
On request 32 6 (19%) 66 3 ( 5%) 0.05
Per physician discretion 32 16 (50%) 66 25 (38%) 0.36
No one screened 32 2 ( 6%) 66 1 ( 2%) 0.25
Screening culture sites
Vagina and rectum & 32 10 (31%) 64 * 48 (75%) <0.01
Vagina and cervix 32 8 (25%) 64 2 ( 3%) <0.01
Vagina only 32 9 (28%) 64 5 ( 8%) 0.01
Cervix only 32 3 ( 6%) 64 2 ( 3%) 0.33
Timing of culture
Recommended time @ 32 7 (22%) 65 * 46 (65%) <0.01
Laboratory isolation methods
for prenatal screening
Selective broth media & 24 14 ( 6%) 161 76 (47%) <0.01
Respondents to both 1994 and 1997
surveys
Any GBS disease prevention
policy 51 18 (35%) 51 29 (57%) 0.21
Written policy 51 8 (16%) 51 21 (41%) <0.01
Recommend selective broth
media 60 3 ( 5%) 60 32 (53%) <0.01
---------------------------------------------------------------------------------------------------------------
* Totals differ from number with intrapartum antibiotic policy or number with screening policy because of
missing data.
+ Risk factors in the 1992 American College of Obstetricians and Gynecologists guidelines (intrapartum fever,
preterm labor, prolonged duration of membrane rupture, or previous infant with GBS disease) for 1994
survey; risk factors in the 1996 consensus guidelines (1992 risk factors and GBS bacteriuria during current
pregnancy) for 1997 survey.
& Recommended by the 1996 consensus guidelines.
@ For 1994 survey, 26-28 weeks' gestation; for 1997 survey, 35-37 weeks' gestation.
=================================================================================================================
Return to top. Figure_2 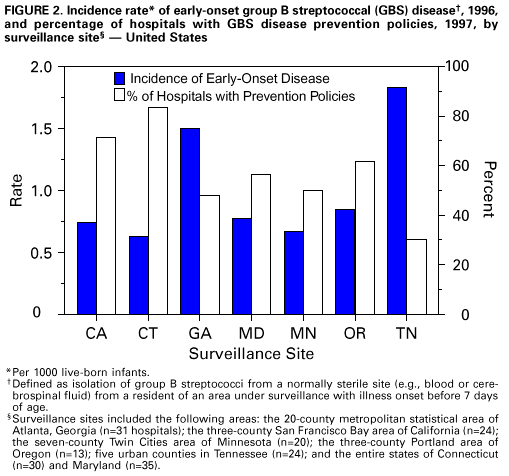 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|