 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Imported Dengue --- United States, 1997 and 1998Dengue is a mosquito-transmitted acute viral disease caused by one of four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). Dengue is endemic in most tropical areas of the world and has occurred in U.S. residents returning from travel to such areas. CDC maintains a laboratory-based passive surveillance system for imported dengue among U.S. residents. The system relies principally on reports by clinicians to state health departments, which forward patient specimens to CDC for diagnostic testing. This report summarizes information about imported dengue cases among U.S. residents for 1997 and 1998, which indicates that most persons with a known travel history probably acquired infection in the Caribbean islands or Asia. Serum samples from 349 persons who had suspected dengue based on clinical presentation and onset of symptoms (1) in 1997 and 1998 were submitted to CDC from 40 states and the District of Columbia. From these samples, 143 (38%) cases were laboratory diagnosed as dengue, 133 (93%) cases had IgM antibody in early convalescent samples or single high titers of IgG antibody in acute serum samples, and 10 (7%) cases had isolation of dengue virus. In three cases, positive by detection of anti-dengue IgM antibody, virus serotype was identified by polymerase chain reaction (PCR). Overall, DEN-4 was identified in five (39%) cases, DEN-2 in four (31%) cases, and DEN-1 and DEN-3 in two (15%) cases each (Table 1). Dengue diagnosis was negative in 129 (37%) patients and indeterminate in 77 (22%) patients because convalescent samples for serologic testing were unavailable. Of the 143 persons with laboratory-diagnosed dengue, sex was known for 130; 65 (50%) were males. Age was reported for 99 persons and ranged from age <1--70 years (median: 34 years). States reporting the highest number of cases were Florida (12) in 1997 and New York (22) in 1998. Travel histories within the 2 weeks before illness, available for 122 persons, indicated that infections probably were acquired in the Caribbean islands (61 cases), Asia (30), Central America (23), South America (four), Africa (three), and the Pacific islands (one). In 1998, 90 laboratory-diagnosed cases were reported, a 70% increase from the 53 cases reported in 1997. Among the 90 cases, 35 (39%) persons reported traveling to the Caribbean islands in 1998 compared with 14 (26%) in 1997. Clinical information was available for 85 patients with laboratory-diagnosed dengue. Commonly reported symptoms were fever (94%), headache (69%), myalgia (53%), rash (53%), arthralgia (32%), retro-orbital pain (27%), nausea or vomiting (25%), chills (24%), diarrhea (19%), and petechiae or ecchymoses (15%). At least seven patients were hospitalized, and one patient died (diagnosed with DEN-2 by immunohistochemistry on autopsy tissue). Reported by: State and territorial health departments. Infectious Disease Pathology Activity, Div of Viral and Rickettsial Diseases; Dengue Br, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, CDC. Editorial Note:The principal vector of dengue is the mosquito Aedes aegypti, which has a wide distribution in most tropical and subtropical areas. In the United States, Ae. aegypti can be found during summer months in many states. Most U.S. residents with dengue become infected during travel to tropical areas, although autochthonous transmission of dengue was documented in Texas in 1999 (2,3). The incubation period of dengue is 4--7 days (range: 3--14 days). Dengue virus infection can be asymptomatic or cause illnesses ranging from mild undifferentiated fever to severe disease, including hemorrhagic manifestations and shock (4). Dengue hemorrhagic fever (DHF) is characterized by fever, minor or major bleeding phenomena, thrombocytopenia (<100,000 platelets/mm3), and evidence of increased vascular permeability (e.g., hemoconcentration [hematocrit increased by at least 20% from baseline], pleural or abdominal effusions, or hypoproteinemia) (4). Dengue shock syndrome (DSS) is DHF with signs of circulatory failure, including narrow pulse pressure (<20 mmHg), hypotension, or shock, and may result in death rates of approximately 10% (5). From 1993 through 1998, the number of imported laboratory-diagnosed U.S. cases increased, reflecting the impact of travel and the occurrence of epidemic activity, especially in the Caribbean and Central America. In 1998, laboratory-diagnosed cases of dengue were more than double the number reported in 1997. This pattern is consistent with the increased number of cases of dengue/DHF in the Americas for 1998 (741,794) compared with 1997 (364,945) (6). The findings in this report are subject to at least two limitations. First, the number of dengue cases referred to CDC for diagnosis represents a minimum estimate of the actual number of U.S. travelers with dengue fever or its complication, DHF or DSS. Because dengue is not a nationally notifiable disease, diagnostic samples may not be sent for testing or may be sent to laboratories other than CDC; therefore, many imported cases may not be counted. For example, Florida implemented an active laboratory-based surveillance system from April 1, 1997, through March 31, 1998, which resulted in an increased detection of laboratory-positive cases from a previous 30-year annual mean of 1.4 cases to 18 cases during this period (7); five of the 18 cases were reported from private clinical laboratories. Second, travel histories and clinical information were not available for all persons with dengue, and they may not be representative of all persons with imported dengue. Persons traveling to areas where dengue is endemic should avoid exposure to mosquitoes by using repellents, wearing protective clothing, and remaining in well-screened or air-conditioned areas. No vaccine is available for preventing dengue infection. The Ae. aegypti mosquito is well adapted to urban environments and can be found in or near human dwellings, where the mosquito can be found in closets, bathrooms, behind curtains, and under beds. The species usually bites during the early morning and late afternoon, but may feed at any time during the day when indoors or during overcast periods (8). With an increase in traveling to and from endemic areas, more cases of imported dengue may be expected and health-care providers should consider dengue in the differential diagnosis of illness for all patients who have fever and a history of travel to tropical areas within 2 weeks before the onset of symptoms. Supportive measures should be given, and only acetaminophen is recommended for management of pain and fever. Acetylsalicylic acid (i.e., aspirin) and other nonsteroidal anti-inflammatory agents are contraindicated because of their anticoagulant properties. Acute-phase and convalescent-phase serum samples should be obtained for viral isolation and diagnosis and sent for confirmation through state or territorial health departments to CDC's Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, 2 Calle Casia, San Juan, PR 00921--3200; telephone (787) 766- 5181; fax (787) 766-6596. Serum samples should be accompanied by a summary of clinical and epidemiologic information, including date of onset of disease, date of collection of sample, and a detailed recent travel history. References
Table 1 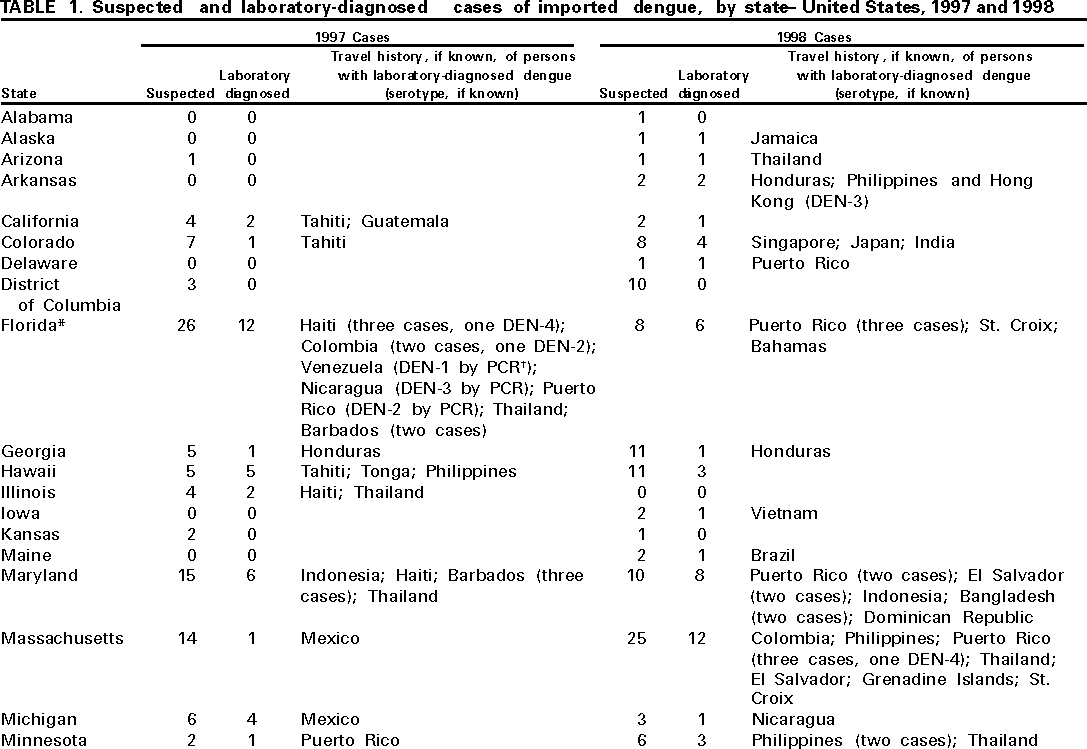 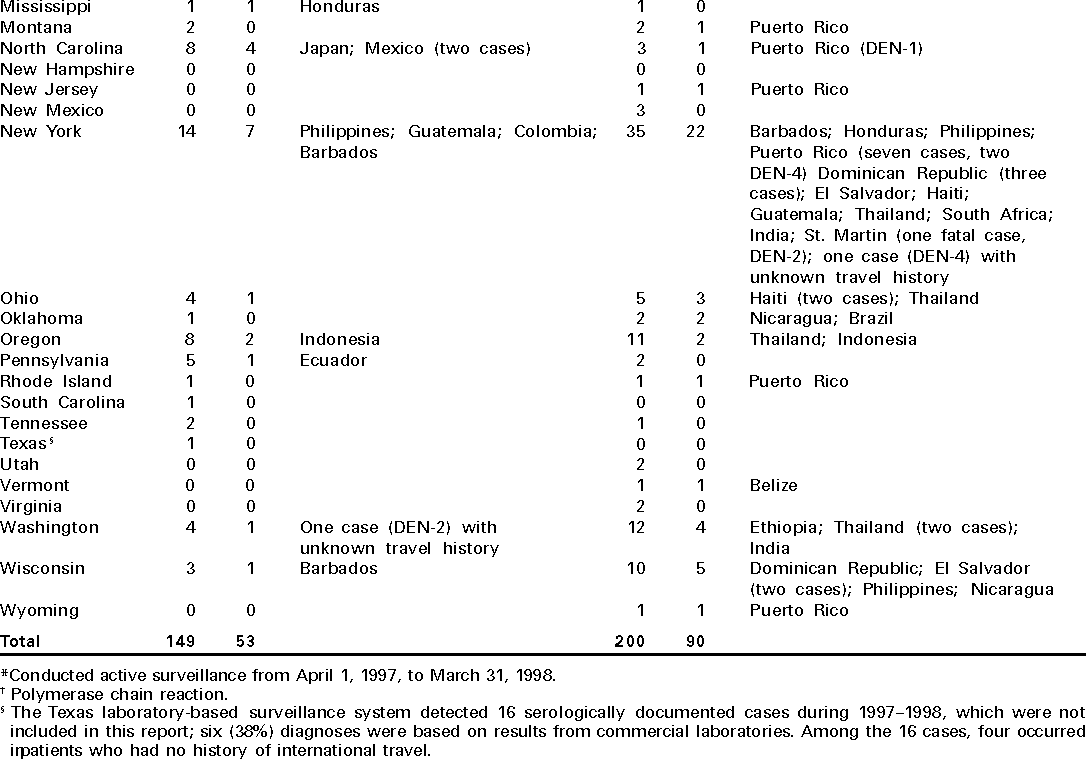 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 3/30/2000 |
|||||||||
This page last reviewed 5/2/01
|