 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
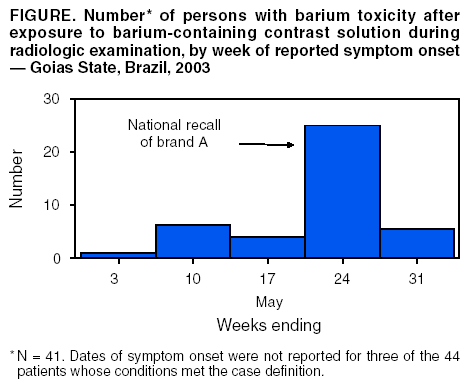
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Barium Toxicity After Exposure to Contaminated Contrast Solution --- Goias State, Brazil, 2003Barium-containing contrast solutions are commonly used in radiologic studies. On May 22, 2003, three patients at radiology clinics in Goias State, Brazil, were hospitalized after ingesting such solutions; two persons died within 24 hours of hospitalization. Exposure occurred during radiologic examination of the upper or lower gastrointestinal tract. An investigation was conducted by municipal and state public health authorities with assistance from the Ministry of Health's National Agency for Sanitary Surveillance (ANVISA) and Brazil's Field Epidemiology Training Program (FETP), known locally as EPISUS. This report summarizes the results of that investigation, which found that 44 persons had suspected barium toxicity (Figure), nine of whom died. Eight of the nine deaths were linked to a single lot of brand A contrast solution. A national recall was announced on May 23, and the manufacturing facility was inspected and closed. Clinicians should be alert for signs of barium toxicity in patients in the hours after administration of contrast solutions during radiologic studies. The field investigation included searches at 15 clinics and hospitals in Goias State that performed radiologic examinations using barium-containing contrast solution. Details were collected regarding contrast administration (i.e., brand, lot number, dose, and route of administration). Hospital charts were reviewed, and interviews were conducted with surviving patients and the family members of children and those patients who died to collect demographic information, medical histories, symptoms, and outcomes. A possible case of barium intoxication in a patient was defined as acute onset of two or more symptoms (i.e., nausea, vomiting, diarrhea, or abdominal pain) occurring <24 hours of undergoing radiologic examination with contrast solution, during April 29--May 31. Of 223 patients in Goias State undergoing radiologic examination with barium-containing contrast solution during the study period, 44 (20%) had suspected toxicity, and 11 (26%) were hospitalized; nine (21%) of the 44 died. Median age of persons with conditions meeting the case definition was 51 years (range: 3 months--97 years); 24 (55%) were female. Contrast solution had been administered orally in 38 (86%) symptomatic persons during evaluations of the esophagus, stomach, or upper gastrointestinal tract, and rectally in six (14%) persons for barium enema study. The median interval between administration and symptom onset was 1.0 hours (range: 0.1 hours-- 5 hours), and all deaths occurred <24 hours of exposure. Among those patients who reported specific symptoms, 40 (93%) of 43 had nausea, 38 (88%) of 43 had abdominal pain, 35 (80%) of 44 had diarrhea, 29 (27%) of 43 had vomiting, 14 (37%) of 38 had headache, 14 (34%) of 41 had dyspnea, 10 (29%) of 35 had cardiac arrhythmias, and 11 (27%) of 40 had agitation. In Goias State, three brands of barium-containing contrast solution were used by radiology clinics during the outbreak period. However, a single lot of brand A contrast solution was associated with eight (89%) of the nine deaths. Although brand A was not administered routinely at the clinic visited by the ninth victim, medical staff believed brand A might have been administered unintentionally after purchase at a local pharmacy. Laboratory testing of unopened containers of the implicated lot of contrast solution showed the concentration of soluble barium was 7,190 + 863 mg/L (mean + 1 s.d.) compared with a reference standard of <5 mg/L (1). In the implicated lot of contrast solution, soluble barium salts (e.g., carbonate or sulfite) were present at approximately 12,370 mg/100 mL (most frequent solution dosage was 150 mL). Previous findings based on animal and human data suggest a lethal oral dose for humans in the range of 2,000 mg--4,000 mg (2). Active searches in other states using the same case definition found that, overall, barium toxicity likely occurred in seven (58%) of the 12 states where brand A was distributed. In states other than Goias, 25 persons were identified with suspected barium toxicity; six (24%) died. A site inspection of the factory producing brand A documented purchases of primary ingredients that were not of pharmaceutical grade. In response, ANVISA revoked the manufacturer's license and forced closure of all facilities. Reported by: RF Silva, National Institute for Quality Control in Health; LQ Santi, AA Santos, MD, F Freitas, Investigation and Prevention of Infection and Adverse Events Unit; MF Dias, MPH, Pharmacological Surveillance Unit; PA Bezerra, National Agency for Sanitary Surveillance; LZ Daufenbach, CP Nascimento, Field Epidemiology Training Program; EH Carmo, MD, Dept of Epidemiological Surveillance; JB da Silva, MD, National Secretariat of Health Surveillance, Ministry of Health, Brazil. DL Hatch, MD, Div of International Health, Epidemiology Program Office, CDC. Editorial Note:Radio-opaque solutions containing barium are used worldwide to provide contrast for diagnostic radiographic examinations, mainly of the gastrointestinal tract (3). Barium sulfate has minimal toxicity when used in contrast solutions because this salt is insoluble in water or lipid and not normally absorbed by the gastrointestinal mucosa. Nevertheless, severe, life-threatening intoxication can occur after ingestion or inhalation of even minute amounts of the absorbable salts of barium (e.g., barium chloride, carbonate, or sulfide) during radiologic examination or in occupational settings (e.g., mining, refining operations, or production of fireworks or rodenticides) (4--7). Brazil's FETP assisted in this investigation. Created in 2000 to establish an experienced core group of epidemiologists in the Ministry of Health, the program has trained 21 epidemiologists to rapidly investigate infectious disease outbreaks, natural disasters, and other events of public health importance. Nausea, vomiting, and profuse watery diarrhea can occur rapidly after exposure to soluble barium salts. Symptoms of intoxication can include severe muscle weakness, respiratory arrest, coma, cardiac arrhythmia, or electrolyte imbalance (e.g., severe hypokalemia) (8--10). Clinicians should watch for signs of barium toxicity in persons receiving contrast solutions during radiologic studies and be prepared to monitor and stabilize cardiorespiratory dysfunction or electrolyte imbalances that might occur rapidly after exposure. In addition, regulators should ensure that only pharmaceutical grade barium sulfate is used in the production of contrast solution for radiologic studies. Acknowledgments This report is based in part on contributions by LC Alencar, MA Vieira, PC Eliam, FM Barbosa, Dept of Sanitary Surveillance, Municipal Secretary of Health, Goiânia; AG Araújo, DD Dias, Center for Toxicological Information; AM Cardoso, SA Silva, GS Mendonça, JF Moraes, MC Brito, Dept of Sanitary Surveillance; LF Tomé, NC Paula, Central Public Health Laboratory; MA Fernandes, VG Albernaz, PC Fonseca, Dept of Epidemiological Surveillance, State Secretariat of Health, Goias State, Brazil. J Stocklin, U.S. Food and Drug Administration, Washington, DC. RL Jones, PhD, J Osterloh, MD, Div of Laboratory Sciences, National Center for Environmental Health, CDC. References
 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/30/2003 |
|||||||||
This page last reviewed 10/30/2003
|