 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Notice to Readers: Updated Recommendations for Use of Pneumococcal Conjugate Vaccine: Reinstatement of the Third DosePlease note: An erratum has been published for this article. To view the erratum, please click here. In February 2004, production of the 7-valent pneumococcal conjugate vaccine (PCV7), marketed as Prevnar® and manufactured by Wyeth Vaccines (Collegeville, Pennsylvania), failed to meet demand, resulting in shortages. To conserve the limited supply, CDC recommended that the fourth dose of PCV7 be withheld from healthy children (1). In March, because evidence indicated that production would be curtailed for several months, CDC recommended that the third dose also be withheld (2). Production problems now appear to have been resolved. As a result, deliveries are projected during the near term to permit the recommendation that every child receive 3 doses. Some providers might have short-term difficulties obtaining vaccine because of distribution delays; however, every effort will be made to provide sufficient vaccine to all providers. Effective immediately, CDC, in consultation with the Advisory Committee on Immunization Practices (ACIP), the American Academy of Family Physicians, and the American Academy of Pediatrics, recommends that providers administer 3 doses of vaccine (3). The fourth dose should still be deferred for healthy children until further production and supply data demonstrate that a 4-dose schedule can be sustained. The full, 4-dose series should continue to be administered to children at increased risk for pneumococcal disease because of certain immunocompromising or chronic conditions (e.g., sickle cell disease, anatomic asplenia, chronic heart or lung disease, diabetes, cerebrospinal fluid leak, and cochlear implant [4]). Alaska Native children and American Indian children who live in Alaska, Arizona, or New Mexico, and Navajo children who live in Colorado and Utah have a risk for invasive pneumococcal disease more than twice the national average. These children should receive the standard 4-dose PCV7 series despite the shortage. An interim catch-up schedule is provided for children who are incompletely vaccinated (Table). The highest priority for catch-up vaccination is to ensure that children aged <5 years at high risk for invasive pneumococcal disease are fully vaccinated. Second priorities include vaccination of healthy children aged <24 months who have not received any doses of PCV7 and vaccination of healthy children aged <12 months who have not yet received 3 doses. Because of the frequency of health-care provider visits by children during their first 18 months, catch-up vaccination might occur at regularly scheduled visits for most children who receive vaccines from their primary-care providers. Programs that provide vaccinations but do not see children routinely for other reasons should consider a notification process to contact undervaccinated children. Wyeth Vaccines is allocating nonpublic-purchased doses of Prevnar® directly to all physicians on the basis of previous purchasing patterns or practice birth cohort. Wyeth does not currently ship products to either wholesalers or distributors. Providers with questions about their allocation or about obtaining Prevnar® should contact Wyeth's customer service department, telephone 800-666-7428. For problems not resolved by the customer service department, providers can contact Wyeth directly, telephone 866-447-8888, extension 37932. For public-purchased vaccine, including Vaccines for Children Program vaccine, providers should contact their state/grantee immunization projects to obtain vaccine. These projects should contact their project officers at the National Immunization Program at CDC for information regarding vaccine supply. This recommendation reflects CDC's assessment of the existing national PCV7 supply and will be changed if the supply changes. Updated information about the national PCV7 supply is available from CDC at http://www.cdc.gov/nip/news/shortages/default.htm. References
Table 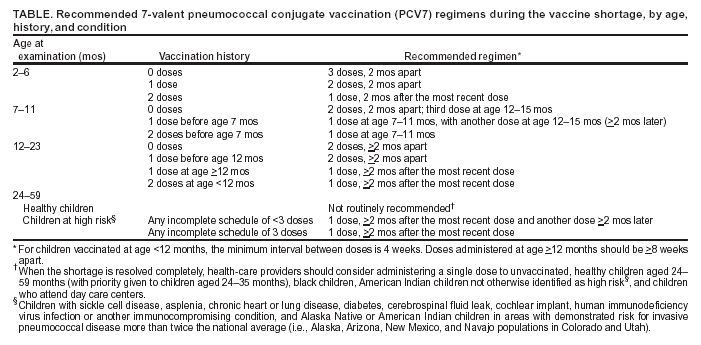 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/7/2004 |
|||||||||
This page last reviewed 7/7/2004
|