Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Influenza Vaccination Coverage Among Children and Adults --- United States, 2008--09 Influenza Season
Before 2008, the Advisory Committee on Immunization Practices (ACIP) had recommended annual vaccination for influenza for persons aged ≥50 years, 18--49 years at higher risk for influenza complications, and 6 months--4 years (1). In 2008, ACIP expanded the recommendations to include all children aged 5--18 years, beginning with the 2008--09 season, if feasible, but no later than the 2009--10 season (2). This expansion added 26 million children and adolescents to groups recommended for routine influenza vaccination. To assess vaccination uptake among children and adults during the 2008--09 influenza season, CDC analyzed data from the Behavioral Risk Factor Surveillance System (BRFSS) in 19 states, which represent 43% of the U.S. population. This report summarizes the results of the analysis, which indicated that reported influenza vaccination coverage of ≥1 doses was 40.9% for ages 6--23 months, 32.0% for 2--4 years, and 20.8% for 5--17 years. Among adults, reported coverage was 32.1% for persons aged 18--49 years with high-risk conditions, 42.3% for persons 50--64 years, and 67.2% for persons ≥65 years. These results are consistent with previous studies that have found no significant increases in vaccination coverage for any of these age groups over previous seasons (1--5).* These 2008--09 season estimates provide a baseline for assessing implementation of the 2008 recommendation for school-aged children. Attaining higher coverage rates likely will require additional vaccination programs in schools and expanded vaccination services in provider offices (6,7).
BRFSS is a state-based, random-digit-dialed telephone survey that collects information from approximately 414,000 randomly selected, noninstitutionalized adults aged ≥18 years.† Data are collected monthly in all 50 states, the District of Columbia (DC), Puerto Rico, the U.S. Virgin Islands, and Guam. Collected data are weighted by age, sex, and race/ethnicity to reflect each state's adult population. To determine influenza vaccination status, respondents were asked, "During the past 12 months, have you had a flu shot?" and "During the past 12 months, have you had a flu vaccine that was sprayed in your nose?" Persons who answered "yes" to either question were asked what month and year their most recent influenza vaccination was received. For the January and February 2009 BRFSS survey conducted just before the beginning of the 2009 H1N1 influenza outbreak, 19§ of the 46 states and DC that were participating volunteered to add two questions to assess seasonal influenza vaccination in children. The questions asked respondents to indicate whether a randomly selected child in each eligible household had received an influenza vaccination within the past 12 months and in what month (for those who had received a vaccination). Weighted data from these 19 states were combined to estimate coverage levels for adults and children for the 2008--09 season. Vaccination coverage estimates are based on vaccinations during August--December.
During the 2008--09 influenza season, the Council of American Survey and Research Organizations (CASRO) state response and cooperation rates¶ (including median and range for each) for these 19 states were 53.7% (37.9--66.1) and 76.7% (57.8--86.4), respectively. Respondents who reported unknown influenza vaccination status (don't know, refused, missing, or blank or incomplete date of vaccination) (4.8%) were excluded from the analysis. Software for statistical analysis of complex survey data was used to calculate point estimates and 95% confidence intervals. Statistical differences between groups were determined using the t-test (p≤0.05).
Seasonal influenza vaccination coverage estimates for adults in the 19 states were 67.2% (ages ≥65 years), 42.3% (50--64 years), 22.2% (18--49 years), and 32.1% (18--49 years, with diabetes, asthma, or heart disease) (Table). Among children, coverage estimates were 40.9% (ages 6--23 months), 32.0% (2--4 years), 20.8% (5--17 years), and 24.0% (6 months--17 years). Among all persons aged ≥6 months, coverage was higher among non-Hispanic whites (36.7%) compared with non-Hispanic blacks (24.9%) (p<0.001) and Hispanics (22.0%) (p<0.001). Age-specific coverage levels were higher among non-Hispanic whites compared with non-Hispanic blacks for the two oldest age groups (50--64 years and ≥65 years) (p=0.002 and p=0.03), and compared with Hispanics for children aged 2--4 years (p<0.001).
During the 2004--05 season, because of a vaccine shortage, BRFSS-estimated coverage levels dropped by 9 percentage points among persons aged ≥65 years, 20 points among persons aged 50--64 years, and 12 points among persons aged 18--49 years with high-risk conditions. Coverage levels among adults for the past four seasons (Figure) have increased to nearly the same levels of those preceding 2005--04 season. The 2008--09 coverage estimates were still lower than those during 2003--04, the season before the vaccine shortage, by 5.3, 3.2, and 4.7 percentage points, respectively, for the ≥65, 50--64, and 18--49 years age groups.
Reported by: GL Euler, DrPH, PJ Lu, PhD, MD, A Shefer, MD, JA Singleton, MS, Immunization Svc Div, A Fiore, MD, Influenza Div, National Center for Immunization and Respiratory Diseases; M Town, MS, L Balluz, ScD, Div of Adult and Community Health, National Center for Chronic Disease Prevention and Health Promotion, CDC.
Editorial Note:
CDC routinely monitors influenza vaccination coverage levels using four data sources. The results in this report come from the nationwide BRFSS surveillance system, used here in 19 states that collected influenza vaccination data for all children aged ≥6 months in 2009. Other sources for monitoring influenza vaccination coverage rates include the National Immunization Survey (NIS), the National Health Interview Survey (NHIS), and eight sentinel immunization information system (IIS) sites located in the United States. These data sources differ in their geographic scope, age groups and population types covered, type of vaccination data, accuracy of reporting, sample representativeness, and timeliness. The special BRFSS survey conducted in early 2009 provided estimates for the 2008--09 season about 1 year earlier than usual, and for children for whom BRFSS has not routinely collected influenza vaccination data.
In 2008, ACIP recommended that all children aged 5--18 years be vaccinated annually for influenza, beginning with the 2008--09 season, if feasible, but no later than the 2009--10 season (2). This report presents findings from the first large-scale, state-based assessment of the response to this recommendation and indicates that approximately 20% of school-aged children were vaccinated during the 2008--09 season. Recent NHIS results demonstrate that influenza vaccine coverage rates among both children and adults were stable over the 2007--08 and 2008--09 seasons.* The national stability found by NHIS supports the use of these first estimates by BRFSS of school-aged influenza vaccination coverage as an overall baseline for gauging future coverage as the states move into the first full season of the new recommendation.
These BRFSS results generally are consistent with other surveys, including prior BRFSS, NIS, and NHIS surveys, which do not indicate significant increases of vaccination coverage in any of these age groups (3--5).¶ Although recently published coverage rates from IIS sentinel sites results (8) are not directly comparable to the 2008--09 BRFSS results in this report (because of differing methods and sources of data [9], varying completeness and accuracy of vaccination histories, and different populations surveyed), they generally corroborate the BRFSS results. The estimated coverage for ≥1doses in this report for children aged 6--23 months (40.9%) is lower than those for the same season in the IIS sentinel sites (47.8%), but estimated coverage in this report is higher for older children, 32.0% versus 27.8% for aged 2--4 years, and 20.8% versus an average of 12.7% for school-aged children.
BRFSS influenza vaccination coverage among adult target groups for the 2008--09 season described in this report were similar to results from prior seasons, and coverage remained below Healthy People 2010 objectives of 60% for high-risk adults aged <65 years and 90% for adults aged ≥65 years (objective 14-29) (3).** Adult coverage levels have remained below those achieved during the 2003--04 season, before the influenza vaccine shortage of 2004--05, highlighting the difficulties in improving coverage above current levels even among adults for whom recommendations are long standing.
The findings in this report are subject to at least six limitations. First, the BRFSS is a landline telephone survey, and therefore subject to selection bias because of noninclusion of cell-phone--only households and households with no telephone service. Second, nonresponse bias might remain after weighting adjustments. Third, the vaccination coverage estimates reported here are based on data from 19 states. Consequently, those estimates might not be representative of the entire U.S. population. However, seasonal influenza vaccination coverage estimates among adults in the 19 states were similar to those for the 46 states and District of Columbia (within 0.2--2.7 points, depending on the age group), and to the NHIS results (5). Fourth, influenza vaccination status was based on self-report, which might result in under- or overreporting because of recall or social desirability bias. Fifth, this survey collected coverage status only through December, although vaccinations continued through March, this underestimates vaccination coverage. However, a comparison using 2008 BRFSS data found that, based on interviews primarily from January and February, coverage among adults was no more than 4 percentage points lower than coverage based on March through August interviews (CDC, unpublished data, 2009). Finally, the BRFSS question about child influenza vaccination asks for the date of the most recent flu vaccination received during the 12 months before the day of the interview; consequently, full vaccination status among children aged 6 months--8 years, who require 2 vaccine doses in their first season to be vaccinated fully, could not be determined.
Reminder and recall systems and standing orders programs have been shown to be effective in all age groups (7). Wider use of these interventions can achieve higher coverage among children and adults recommended for influenza vaccination (1). Vaccination programs in schools and other community settings supplementing vaccination services routinely provided in health-care provider offices and public health clinics (1,6,7) also can increase coverage.
References
- CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-8).
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR 2008;57(No. RR-7).
- CDC. State-specific influenza vaccination coverage among adults---United States, 2006--07 influenza season. MMWR 2008;57:1033--9.
- CDC. Influenza vaccination coverage among children aged 6--23 months---United States, 2007--08 influenza season. MMWR 2009;58:1063--6.
- CDC. Vaccination coverage estimates from the National Health Interview Survey: United States, 2008. (Updated July 22, 2009.) Available at http://www.cdc.gov/nchs/data/hestat/vaccine_coverage.htm.
- Rand CM, Szilagyi PG, Yoo BK, Auinger P, Albertin C, Coleman MS. Additional visit burden for universal influenza vaccination of U.S. school-aged children and adolescents. Arch Pediatr Adolesc Med 2008;162:1048--55.
- Pickering LK, Baker CJ, Freed GL, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis 2009;49:817--40.
- CDC. Influenza vaccination coverage among children aged 6 months--18 years---eight immunization information system sentinel sites, United States, 2008--09 influenza season. MMWR 2009;58:1059--62.
- Shinall M, Plosa EJ, Poehling KA. Validity of parental report of influenza vaccination in children aged 6 to 59 months of age. Pediatrics 2007; 120:e783--7.
* CDC. Early release of selected estimates based on data from the January--March 2009 and the January--March 2008 National Health Interview Survey receipt of influenza vaccination. Available, respectively, at http://www.cdc.gov/nchs/data/nhis/earlyrelease/200909_04.pdf and at http://www.cdc.gov/nchs/data/nhis/earlyrelease/200809_04.pdf.
† Additional information and survey questions available at http://www.cdc.gov/brfss.
§ Alaska, California, Connecticut, Delaware, Hawaii, Illinois, Iowa, Kansas, Maine, Michigan, Nevada, New Mexico, Ohio, Texas, Utah, Washington, West Virginia, Wisconsin, and Wyoming.
¶ The CASRO response rate is the product of three other rates: the resolution rate, which is the proportion of telephone numbers that can be identified as either for a business or a residence; the screening rate, which is the proportion of qualified households that complete the screening process; and the cooperation rate, which is the proportion of contacted eligible households for which a completed interview is obtained.
** CDC data for the 2007--08 season were in preparation for publication at the time of this report.
FIGURE. Estimated influenza vaccination coverage among persons aged ≥18 years --- United States, Behavioral Risk Factor Surveillance System (BRFSS), 1992--93 through 2008--09 influenza seasons*
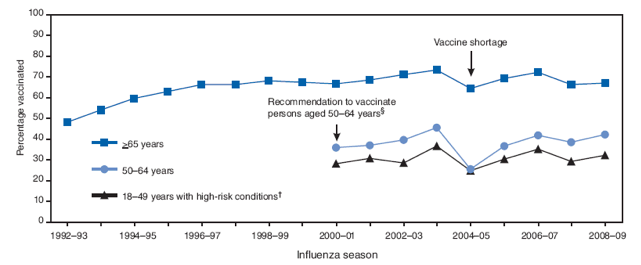
* Data for the 2008--09 season were obtained from a survey conducted in 46 states and the District of Columbia, primarily during January and February 2009, and include vaccinations given during August--December 2008. Data for the 2007--08 season are based on February--August interviews only and vaccinations given during September 2007--January 2008. All other data points are based on February--August interviews only and vaccinations given in the preceding 12 months of interview.
† Persons who had asthma or diabetes were identified as having high-risk conditions for the 2000--01 through 2004--05 seasons, and persons with asthma, diabetes, or heart diseases were identified as having high-risk conditions for the 2005--06 through 2008--09 seasons.
§ The Advisory Committee on Immunization Practices added a recommendation to vaccinate all persons aged 50--64 years, beginning with the 2000--01 influenza season. BRFSS also began collecting influenza vaccination data in the 2000--01 influenza season for persons aged 50--64 years and for persons aged 18--49 years with selected high-risk conditions.
Alternative Text: The figure above shows the estimated influenza vaccination coverage among persons aged ≥18 years in the United States from the 1992-93 through 2008-09 influenza seasons, derived from the Behavioral Risk Factor Surveillance System (BRFSS). BRFSS-estimated coverage levels have varied little among adults for the past four seasons. During the 2004-05 season, because of a vaccine shortage, coverage dropped by 9 percentage points among persons aged ≥65 years, 20 points among persons aged 50-64 years, and 12 points among persons aged 18-49 years with high-risk conditions. The 2008-09 coverage estimates were still lower than those during 2003-04, the season before the vaccine shortage, by 5.3, 3.2, and 4.7 percentage points, respectively, for the >65, 50-64, and 18-49 years age groups.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 10/8/2009


