Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Seroprevalence of Herpes Simplex Virus Type 2 Among Persons Aged 14--49 Years --- United States, 2005--2008
Herpes simplex virus type 2 (HSV-2) is one of the most common sexually transmitted infections worldwide and the primary cause of genital and neonatal herpes and genital ulcer disease (1). Multiple studies have shown that HSV-2 infection increases the risk for human immunodeficiency virus (HIV) infection by at least twofold (2). HSV-2 infection is lifelong, and serologic testing provides the best method to estimate HSV-2 prevalence. Since 1976, CDC has monitored HSV-2 seroprevalence in the United States through the National Health and Nutrition Examination Survey (NHANES). After increasing from 1976--1980 (NHANES II) to 1988--1994 (NHANES III), HSV-2 seroprevalence decreased, from 21.0% in 1988--1994 to 17.0% in NHANES 1999--2004 (1). To determine whether HSV-2 seroprevalence in the United States has changed since 1999--2004 and to estimate HSV-2 seroprevalence by age, race/ethnicity, and reported lifetime number of sex partners, CDC analyzed serologic test results from persons aged 14--49 years who participated in NHANES 2005--2008. The results indicated that HSV-2 seroprevalence was 16.2% overall, not statistically different from the seroprevalence in 1999--2004. Seroprevalence was highest among women (20.9%) and non-Hispanic blacks (39.2%). Of those infected with HSV-2, 81.1% had not received a diagnosis. Clinicians, health departments, health-care organizations, and community groups should promote measures that prevent HSV-2 transmission, including minimizing the number of sex partners, avoiding concurrent sexual partnerships, and using condoms consistently and correctly. Patients with known HSV-2 infection should be tested for HIV.
NHANES surveys are cross-sectional surveys designed to compile nationally representative statistics on the health of the U.S. civilian, noninstitutionalized population through complex, multistage probability sampling. During NHANES 2005--2008, a total of 8,283 persons aged 14--49 years were interviewed. Of these, 8,002 had a medical examination; sera were collected from 7,293 participants (88% of those interviewed) for testing of HSV-2 antibodies using a type-specific immunodot assay. Seroprevalence was analyzed by sex, age group, number of lifetime sex partners, and three racial/ethnic categories (non-Hispanic white, non-Hispanic black, and Mexican American). Participants also were asked, "Has a doctor or other health-care professional ever told you that you had genital herpes?" Statistical software was used to generate seroprevalence estimates and 95% confidence intervals. All seroprevalence estimates were weighted using medical examination weights of the survey to represent the U.S. civilian, noninstitutionalized population, accounting for survey participants' unequal probabilities of selection and adjustments for nonresponse. Differences in seroprevalence among categories of participants (e.g., sex or age group) were assessed using the Wald chi-square test.
Overall HSV-2 seroprevalence from NHANES 2005--2008 was 16.2%, not statistically different from the 17.0% seroprevalence in NHANES 1999--2004 (p = 0.34). Of those testing positive for HSV-2 infection, 81.1% said they had never been told by a doctor or health-care professional that they had genital herpes. Seroprevalence increased with age, ranging from 1.4% among those aged 14--19 years to 26.1% among those aged 40--49 years (p<0.001) (Table). HSV-2 seroprevalence was greater among women (20.9%) than men (11.5%) (p<0.001).
By race/ethnicity, HSV-2 seroprevalence was approximately three times greater among non-Hispanic blacks (39.2%) as among non-Hispanic whites (12.3%) (p<0.001). Seroprevalence was greatest among non-Hispanic blacks in the 30--39 years (56.2%) and 40--49 years (56.0%) age groups (Figure 1). In contrast, seroprevalence was 20.8% for non-Hispanic whites aged 40--49 years, and 20.4% for Mexican Americans aged 40--49 years. For all three racial/ethnic groups, HSV-2 seroprevalence generally was greater among those with more lifetime sex partners (Figure 2). For example, among persons with two to four lifetime sex partners, seroprevalence was 9.1% for non-Hispanic whites, 34.3% for non-Hispanic blacks, and 13.0% for Mexican Americans. Among persons with 10 or more lifetime sex partners, seroprevalence was 22.7% for non-Hispanic whites, 49.9% for non-Hispanic blacks, and 17.1% for Mexican Americans. (Figure 2).
Reported by
F Xu, MD, PhD, MR Sternberg, PhD, SL Gottlieb, MD, SM Berman, MD, LE Markowitz, MD, Div of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; SE Forhan, MD, LD Taylor, DrPH, EIS officers, CDC.
Editorial Note
HSV-2 infection is an important public health concern because of the morbidity associated with symptomatic infection, the potential for high rates of clinical and subclinical recurrences, and the strong association between HSV-2 and HIV infections; genital HSV-2 infection in pregnant women can lead to serious infection in neonates through vertical transmission. This report found an overall HSV-2 seroprevalence of 16.2% for the period 2005--2008, not statistically different from the 17.0% seroprevalence found for 1999--2004. As observed in previous surveys (1), CDC found large racial/ethnic disparities in HSV-2 seroprevalence. Most notably, HSV-2 seroprevalence among non-Hispanic blacks was approximately three times that of non-Hispanic whites and nearly four times that of Mexican Americans. This racial disparity is particularly important because non-Hispanic blacks also are at greater risk for HIV acquisition, making up 12% of the adult and adolescent U.S. population but accounting for 46% of persons living with HIV (3).
Persons infected with HSV-2 are at greater risk for HIV acquisition, even in the absence of HSV-2 symptoms (2). Increased susceptibility to HIV infection likely occurs because even HSV-2 ulcerations that are microscopic can provide a portal of entry for HIV, and HSV-2 reactivation recruits potential target cells for HIV to the genital skin and mucosa. Two large randomized controlled trials among persons who were HSV-2-infected and HIV negative found that daily antiviral (acyclovir) therapy to suppress HSV-2 infection did not decrease the risk for HIV acquisition (4). Thus, primary prevention of HSV-2 infection might be the only available strategy to reduce the increased risk for HIV infection associated with HSV-2. Increasing awareness of the high HSV-2 prevalence in the United States and the link between HSV-2 and HIV infections are important first steps in addressing the increased risk for HIV infection, especially among persons at greatest risk for HSV-2 and HIV infections.
This report found that 81.1% of HSV-2 infections were asymptomatic or unrecognized. Viral shedding and transmission to sex partners can occur in the absence of symptoms or a noticeable lesion (5). Multiple prevention strategies used alone or in combination are potentially useful in limiting acquisition and transmission of HSV-2. For persons with symptomatic HSV-2 infection, daily antiviral therapy has been shown to reduce clinical and subclinical reactivation rates of HSV-2 and to reduce the risk for HSV-2 transmission to uninfected partners by 50% (6).
CDC recommends that persons with genital herpes avoid sexual activity with uninfected partners when lesions or prodromal symptoms are present because the greater viral shedding that accompanies these symptoms likely increases risk for HSV-2 transmission (5). Infected persons also should be counseled regarding the HSV-2 transmission risk associated with viral shedding in the absence of symptoms. Latex condoms, when used consistently and correctly, can reduce HSV-2 transmission. A recent meta-analysis found that persons who reported always using condoms had a 30% decreased risk for acquiring HSV-2 infection compared with persons who reported no condom use (7). Additionally, persons with HSV-2 infection should be encouraged to inform partners of their infection status before initiating a sexual relationship.
The findings in this report are subject to at least three limitations. First, NHANES surveys do not assess HSV-2 seroprevalence in military or institutionalized populations; therefore, the estimates are not representative of the entire U.S. population. Second, no information was collected regarding genital symptoms to assess the extent of symptomatic but undiagnosed HSV-2 infection. Finally, because of insufficient sample size, coinfection with HSV-2 and HIV could not be assessed. However, a previous report using data from multiyear NHANES found a strong association between HSV-2 seropositivity and HIV infection in the general population (8).
Persons with known HSV-2 infection should be tested for HIV and adopt HIV risk-reduction strategies, such as limiting the number of sex partners and using condoms consistently and correctly. However, in this analysis, 81.1% of seropositive participants reported never having received a diagnosis of HSV-2 infection. A substantial proportion of these persons might have nonspecific signs (e.g., small fissures or localized erythema) or mild symptoms that are not recognized as genital herpes (9). Therefore, health-care providers should consider testing for HSV-2 infection in those patients with either typical or atypical genital complaints, especially when the symptoms are recurrent (5).
Routine serologic testing might identify numerous unrecognized HSV-2 infections; however, the role of routine serologic screening in population-based prevention efforts has been controversial (10). Serologic HSV-2 testing is not recommended currently for the general population because of concerns related to test performance in low-prevalence settings and a lack of data regarding the benefits of screening (10). Nonetheless, HSV serologic screening might be useful in selected populations at high risk, such as persons with multiple sex partners or HIV infection, and men who have sex with men (5). Additional research is needed to determine the benefits, feasibility, and cost effectiveness of serologic testing, alone or in combination with antiviral treatment, to prevent HSV-2 acquisition and transmission (10). Finally, research into development of an HSV-2 vaccine should continue and might result in a more effective preventive measure in the future.
References
- Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006;296:964--73.
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006;20:73--83.
- CDC. Subpopulation estimates from the HIV Incidence Surveillance System---United States, 2006. MMWR 2008;57:985--9.
- Celum C, Wald A, Hughes J, et al. Effect of acyclovir on HIV-1 acquisition in herpes simplex virus type 2 seropositive women and men who have sex with men: a randomized, double blind, placebo-controlled trial. Lancet 2008;371:2109--19.
- CDC. Sexually transmitted disease treatment guidelines. MMWR 2006;55(No. RR-11).
- Corey L, Wald A, Patel R, et al; Valacyclovir HSV Transmission Study Group. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004;350:11--20.
- Martin ET, Krantz E, Gottlieb SL, et al. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med 2009;169:1233--40.
- McQuillan GM, Kruszon-Moran D, Granade T, Feldman JW. Seroprevalence of human immunodeficiency virus in the US household population aged 18--49 years: the National Health and Nutrition Examination Surveys, 1999--2006. J Acquir Immune Defic Syndr 2010;53:117--23.
- Langenberg A, Benedetti J, Jenkins J, Ashley R, Winter C, Corey L. Development of clinically recognizable genital lesions among women previously identified as having "asymptomatic" herpes simplex virus type 2 infection. Ann Intern Med 1989;110:882--7.
- Douglas J, Berman S. Screening for HSV-2 infection in STD clinics and beyond: a few answers but more questions. Sex Transm Dis 2009;36:729--31.
What is already known on this topic?
Herpes simplex virus type 2 (HSV-2) is a common sexually transmitted infection in the United States and is associated with an increased risk for human immunodeficiency virus (HIV) infection.
What is added by this report?
During 2005--2008, HSV-2 seroprevalence was 16.2% among persons aged 14--49 years in the United States and higher (39.2%) among non-Hispanic blacks; 81.1% of infected persons had not received a diagnosis.
What are the implications forpublic health practice?
Health-care providers should consider HSV-2 infection in the differential diagnosis of genital complaints consistent with HSV-2 infection; patients with known HSV-2 infection should be tested for HIV. Clinicians, health departments, health-care organizations, and community groups should promote measures that prevent HSV-2 transmission, including minimizing the number of sex partners, avoiding concurrent sexual partnerships, and using condoms consistently and correctly.
FIGURE 1. Herpes simplex virus type 2 seroprevalence* among persons aged 14--49 years, by age group and race/ethnicity† --- National Health and Nutrition Examination Survey, United States, 2005--2008
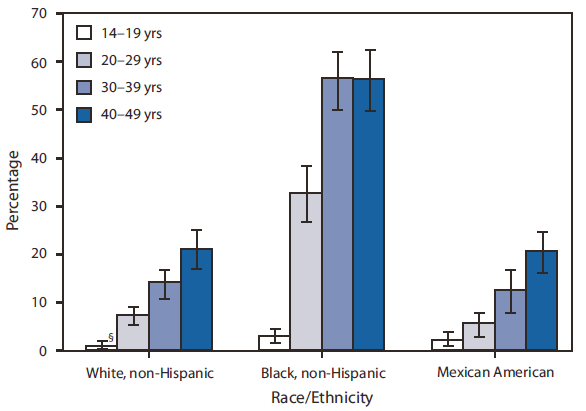
* All seroprevalence estimates were weighted using medical examination weights of the survey to represent the U.S. civilian, noninstitutionalized population, accounting for survey participants' unequal probabilities of selection and adjustments for nonresponse.
† The categories black, non-Hispanic and white, non-Hispanic include persons who reported only one race and exclude persons of Hispanic ethnicity. Persons of Mexican American ethnicity might be of any race.
§ 95% confidence interval.
Alternate Text: The figure above shows herpes simplex virus type 2 seroprevalence among persons aged 14-49 years in the United States during 2005-2008, by age group and race/ethnicity. By race/ethnicity, HSV-2 seroprevalence was approximately three times greater among non-Hispanic blacks (39.2%) than non-Hispanic whites (12.3%) (p<0.001). Seroprevalence was greatest among non-Hispanic blacks in the 30-39 years (56.2%) and 40-49 years (56.0%) age groups.
FIGURE 2. Herpes simplex virus type 2 seroprevalence* among persons aged 14--49 years who reported having had sex, by number of lifetime sex partners and race/ethnicity† --- National Health and Nutrition Examination Survey, United States, 2005--2008
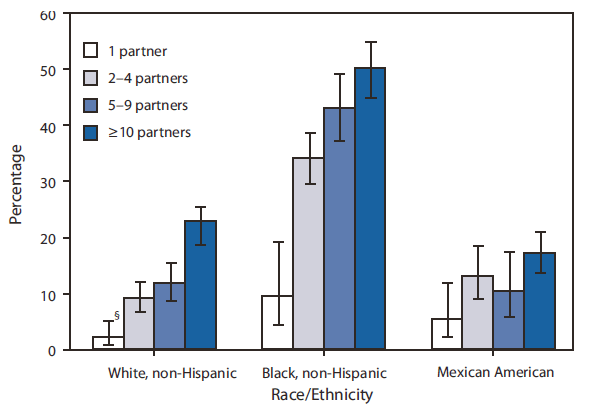
* All seroprevalence estimates were weighted using medical examination weights of the survey to represent the U.S. civilian, noninstitutionalized population, accounting for survey participants' unequal probabilities of selection and adjustments for nonresponse.
† The categories black, non-Hispanic and white, non-Hispanic include persons who reported only one race and exclude persons of Hispanic ethnicity. Persons of Mexican American ethnicity might be of any race.
§ 95% confidence interval.
Alternate Text: The figure above shows herpes simplex virus type 2 seroprevalence among persons aged 14-49 years, in the United States from 2005-2008, who reported having had sex, by number of lifetime sex partners and race/ethnicity. Seroprevalence was 20.8% for non-Hispanic whites aged 40-49 years, and 20.4% for Mexican Americans aged 40-49 years. For all three racial/ethnic groups, HSV-2 seroprevalence generally was greater among those with more lifetime sex partners.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


