Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Travel-Associated Dengue Surveillance --- United States, 2006--2008
Dengue is caused by four antigenically related viruses (DENV-1, DENV-2, DENV-3, and DENV-4). Dengue fever is endemic in most tropical and subtropical areas of the world, and in 2007 nearly 1 million cases were reported in the Americas alone. Dengue infections commonly occur among U.S. residents returning from travel to endemic areas (1--4) and are more prevalent than malaria among returning travelers from the Caribbean, South America, South Central Asia, and Southeast Asia (5). This report summarizes information about dengue cases reported to CDC through two CDC-maintained passive surveillance systems: 1) the ArboNET surveillance system, a national CDC arboviral surveillance system maintained by CDC's Arboviral Diseases Branch and initially developed in response to the introduction of West Nile virus in the United States, and 2) a system maintained for decades by the CDC Dengue Branch (CDCDB), which collects information on all suspected dengue cases whose specimens are sent to the branch. During 2006--2008, a total of 1,125 unique reports were made to either ArboNET or CDCDB. Of these, the highest proportion of laboratory-confirmed and probable cases with known travel histories were in persons who reported travel to the Dominican Republic (121; 20%), Mexico (55; 9%), and India (43; 7%). Health-care providers should consider dengue in the differential diagnosis of patients with a history of travel to endemic areas within 14 days of fever onset.
Dengue cases are reported to ArboNET from state and metropolitan health departments. The dengue surveillance system at CDCDB receives reports of suspected dengue cases among U.S. travelers from clinicians and officials at state health departments, who forward patient specimens to CDC for diagnostic testing. Age, sex, birth date, and onset date are used to match cases reported to both systems, and duplicate cases are eliminated.
For both CDCDB and ArboNET, a case of laboratory-diagnosed infection (i.e., probable or laboratory-confirmed) in a resident of one of the 50 states or the District of Columbia (DC) who traveled in the 14 days before symptom onset to a dengue-endemic area outside the 50 states. Because autochthonous dengue transmission in the continental United States is uncommon, all cases reported to ArboNET or CDC are classified as travel-associated unless they are identified specifically as locally acquired.
Cases submitted to ArboNET are classified as probable or laboratory-confirmed by the reporting jurisdiction; specimens submitted to CDC are classified as probable or laboratory-confirmed based on testing conducted at CDCDB. Probable cases are defined by a single immunoglobulin M (IgM) specimen in late acute phase or convalescent phase of illness. Laboratory-confirmed cases are defined by a positive polymerase chain reaction (PCR) test result or by viral isolation.
During 2006--2008, 57 duplicate reports were reported to both ArboNET and CDCDB and were assigned to CDCDB. During that period, ArboNET received reports of 596 cases, of which 468 (79%) were reported as probable and 128 (21%) were reported as laboratory-confirmed.
During 2006--2008, CDCDB received a total of 529 specimens from 524 patients in 41 states and DC for dengue testing (153 in 2006, 272 in 2007, and 104 in 2008). Of the 529 specimens, 136 (26%) resulted in a diagnosis of dengue. Among those 136 specimens, 106 (78%) had elevated antidengue IgM antibodies (probable recent dengue infection), and 30 (22%) had a dengue virus identified in serum by either reverse transcription--polymerase chain reaction (RT-PCR) or viral isolation (confirmed acute dengue infection). Serotype specific data were available for those 30 cases, of which 14 were DENV-1, seven were DENV-2, six were DENV-3, and three were DENV-4. Results for 162 (31%) specimens were classified as indeterminate because blood samples were not collected within specified timeframes. Among the 215 patients (41% of all specimens received) whose laboratory results were negative (RT-PCR or IgM negative, or no virus isolated), 38 (18%) had evidence of past flavivirus infection. In addition, the amount of serum provided for 16 (3%) of the patients was insufficient for testing, and in one sample the infecting virus could not be identified.
The 596 case reports were received by ArboNET from 25 states; more than half (57%) were reported from three states: 178 (30%) from New York, 99 (17%) from Florida, and 61 (10%) from Texas. Among the 136 dengue-positive cases identified by CDCDB, 42 (31%) were submitted from New York, 17 (13%) from Massachusetts, 10 (7%) from Arizona, and 10 (7%) from Georgia. Males accounted for 52% of all cases reported to ArboNET and 54% of positive specimens to CDCDB. Median age of patients was similar for both systems; 40 years and 42 years, respectively.
Of the 732 confirmed and probable cases from ArboNET and CDCDB combined (596 cases from ArboNET and 136 positive cases from CDC), history of travel was reported by 649 persons (89%), among whom country-specific travel information was available for 613 (95%). By region, 262 persons (43%) had traveled to the Caribbean; 208 (34%) to Mexico, Central America, or South America; 131 (21%) to Asia and the Pacific; and 12 (2%) to Africa. By country, 121 persons (20%) reported travel to the Dominican Republic, 55 (9%) to Mexico, and 43 (7%) to India during the 14 days before illness onset (Table 1). One laboratory-confirmed case reported to ArboNET from Texas in 2008 was characterized as "not imported," with no travel history, and might have represented autochthonous dengue transmission.
For ArboNET cases, the type of clinical syndrome was recorded in 596 cases; 429 (72%) were categorized as uncomplicated fever, 95 (16%) as dengue hemorrhagic fever or dengue shock syndrome, 56 (9%) as other syndrome or unknown, and 16 (3%) as dengue with hemorrhage (Table 2). For CDCDB cases, clinical syndrome was recorded for 54 (40%) of 136 laboratory-diagnosed cases; 32 (59%) were classified as dengue fever, four (7%) cases as dengue hemorrhagic fever, and no cases of dengue shock syndrome. The most frequently reported signs and symptoms included 77 (57%) cases with fever, 57 (42%) with headache, and 55 (40%) with body ache. Among the 136 CDCDB cases, 27 (20%) included at least one hemorrhagic symptom (e.g., petechiae, purpura, hemoptysis, hematemesis, hematuria, vaginal bleeding, bleeding gums, or epistaxis). A higher proportion of cases submitted to ArboNET (292; 49%) resulted in hospitalization compared with CDCDB cases (26; 19%). One travel-associated dengue fatality was laboratory confirmed by CDCDB.
Reported by
R Luce, DVM, A Rivera, MS, H Mohammed, PhD, KM Tomashek, MD; Dengue Br, J Lehman, Arboviral Diseases Br, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC.
Editorial Note
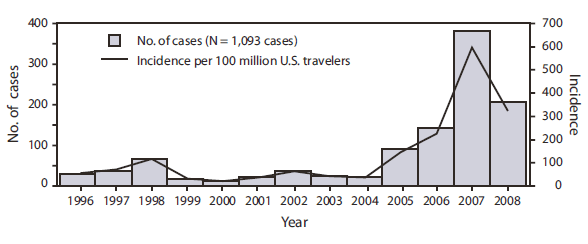
During 2006--2008, an average of 244 confirmed and probable travel-associated dengue cases were identified by ArboNET or CDCDB annually (Figure), compared with an annual average of 33.5 cases (range:13--77 cases) identified during 1990--2005. Most of this increase likely resulted from the 2003 addition of dengue reporting to the ArboNET surveillance system, which supplements CDCDB (6). However, a portion of the increase also likely resulted from substantial increases in dengue incidence throughout subtropical and tropical areas of the world, including the Americas (6). During 2006--2008, dengue outbreaks were reported in numerous countries, including Belize, Brazil, Costa Rica, Cuba, Ecuador, El Salvador, Guadeloupe, India, Madagascar, Martinique, Mexico, Nicaragua, Pakistan, Paraguay, the United States (Puerto Rico, U.S. Virgin Islands), Venezuela, and multiple island nations in the South Pacific. Most U.S. residents become infected during travel to tropical and subtropical areas outside the continental United States, although autochthonous transmission has been documented on multiple occasions since 1980 in Texas (7,8), during 2001--2002 in Hawaii (9), and during 2009--2010 in Florida (10).
Dengue has an incubation period of 3--14 days. Because U.S. travelers spend a median of 10 nights abroad, many returning travelers who are infected could be viremic and able to infect endemic Aedes spp. vector mosquitoes (principally Ae. aegypti and Ae. albopictus) in some locations in the continental United States, thus creating the potential for localized dengue transmission. Clinically recognized cases of travel-associated dengue likely underestimate the risk for importation because many dengue infections are asymptomatic or mildly symptomatic (Box).
The findings in this report are subject to at least three limitations. First, these surveillance results likely are subject to underreporting because both CDCDB and ArboNET are based on passive reporting (i.e., each rely on public health jurisdictions and health-care providers to detect and report infection) and dengue was designated a nationally notifiable disease in the United States in 2010, after the ending date of this report. Second, cases submitted to ArboNET were classified as either probable or laboratory-confirmed by the reporting jurisdiction largely based on interpretation of laboratory results from private laboratories using several different laboratory diagnostic tests, which might have affected classification and reporting of results. Finally, travel histories and clinical information were not available for all cases and might not have been representative of all persons with travel-associated dengue.
Travelers to tropical areas can reduce their risk for dengue by avoiding exposure to mosquitoes. No vaccine is available for preventing dengue infection. Persons traveling to areas where dengue is endemic should use insect repellents, wear protective clothing, and reside in facilities with screens and air conditioning when available. Preventing travel-associated dengue not only benefits the traveler, but also helps prevent the introduction of dengue virus into tropical areas and subtropical areas of the United States (primarily the southeastern states), where vector mosquitoes could transmit the virus indigenously.
Acknowledgments
This report is based, in part, on contributions by state and local health departments, and by surveillance staff members of the Arboviral Diseases Br and the Dengue Br at CDC.
References
- Rigau-Pérez JG, Gubler DJ, Vorndam AV, Clark GG. Dengue: a literature review and case study of travelers from the United States, 1986--1994. J Travel Med 1997;4:65--71.
- CDC. Travel-associated dengue---United States, 2005. MMWR 2006;55:700--2.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Eng J Med 2006;354:119--30.
- CDC. Dengue fever among U.S. travelers returning from the Dominican Republic---Minnesota and Iowa, 2008. MMWR 2010;59:654--6.
- Gubler DJ. Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem. In: Gubler DJ, Kuno G, eds. Dengue and dengue hemorrhagic fever. Wallingford, United Kingdom: CABI International; 1997:1--22.
- Mohammed HP, Ramos MM, Rivera A, et al. Travel-associated dengue infections in the United States, 1996--2005. J Travel Med 2010;17:8--14.
- CDC. Imported dengue---United States, 1999 and 2000. MMWR 2002;51:281--3.
- CDC. Dengue hemorrhagic fever---U.S.--Mexico border, 2005. MMWR 2007;56:785--9.
- Effler P, Pang L, Kitsutani P, et al. An outbreak of dengue fever in Hawaii. Emerg Infect Dis 2005;11:742--9.
- CDC. Locally acquired dengue---Key West, Florida, 2009--2010. MMWR 2010;59:577--81.
What is already known on this topic?
Dengue infections commonly occur among U.S. residents returning from travel to endemic areas and are more prevalent than malaria among returning travelers from the Caribbean, South America, South Central Asia, and Southeast Asia.
What is added by this report?
During 2006--2008, an average of 244 confirmed and probable travel-associated dengue cases annually were identified by two CDC-maintained passive surveillance systems, substantially more than the 33.5 cases (range: 13--77 cases) identified annually during 1990--2005.
What are the implications for public health practice?
Health-care providers should consider dengue in the differential diagnosis of patients with a history of travel to endemic areas within 14 days of fever onset.
|
TABLE 1. (Continued) Cases* of imported dengue reported to ArboNET and CDC Dengue Branch (CDCDB), by state, 2006--2008† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Reported to CDCDB |
Reported to ArboNET |
Total |
|||||||
|
Case classification |
Travel history |
Case classification |
Travel history |
Case classification |
|||||
|
State |
Probable§ |
Laboratory-confirmed¶ |
Travel destinations of laboratory-confirmed dengue (No. of laboratory-positive specimens and serotype, if available)** |
Probable§ |
Laboratory-confirmed¶ |
Travel destinations for laboratory-confirmed dengue** |
Probable§ |
Laboratory-confirmed¶ |
|
|
Oregon |
1 |
0 |
0 |
0 |
1 |
0 |
|||
|
Pennsylvania |
0 |
1 |
Unknown (DENV-3) |
13 |
8 |
Bangladesh, Honduras, Jamaica, Puerto Rico (3), St. Martin, Thailand |
13 |
9 |
|
|
Rhode Island |
0 |
0 |
0 |
0 |
0 |
0 |
|||
|
South Carolina |
0 |
0 |
0 |
1 |
Puerto Rico |
0 |
1 |
||
|
South Dakota |
1 |
0 |
2 |
1 |
Haiti |
3 |
1 |
||
|
Tennessee |
0 |
0 |
0 |
0 |
0 |
0 |
|||
|
Texas |
6 |
1 |
Maldives (DENV-2) |
22 |
39 |
Bangladesh (2), Brazil (2), El Salvador (2), Guatemala, Honduras (2), India (6), Malaysia, Mexico (6), Pakistan, Panama, Paraguay, Philippines, Puerto Rico (5), Singapore (2),Tahiti, Thailand, unknown (2), Venezuela (2) |
28 |
40 |
|
|
Utah |
0 |
0 |
0 |
0 |
0 |
0 |
|||
|
Vermont |
0 |
0 |
0 |
0 |
0 |
0 |
|||
|
Virginia |
0 |
1 |
St. Barthelemy and St. Thomas (DENV-4) |
25 |
5 |
Costa Rica, El Salvador, Jamaica, Puerto Rico, St. Thomas |
25 |
6 |
|
|
Washington |
0 |
0 |
21 |
7 |
El Salvador, Honduras, Nicaragua (3), Philippines, Sri Lanka |
21 |
7 |
||
|
West Virginia |
0 |
0 |
2 |
0 |
2 |
0 |
|||
|
Wisconsin |
1 |
0 |
4 |
16 |
Costa Rica (2), Granada, Guatemala and Mexico, Honduras, Laos, Mexico (3), Nicaragua, Nigeria, Puerto Rico (2), St. Lucia, Saudi Arabia, Thailand |
5 |
16 |
||
|
Wyoming |
1 |
1 |
Ecuador (DENV-4) |
0 |
0 |
1 |
1 |
||
|
Total |
106 |
30 |
468†† |
128§§ |
574 |
158 |
|||
|
* Includes probable and laboratory-confirmed cases. † CDCDB received a total of 529 specimens. The 596 total cases reported to ArboNET are the sum of probable (468) and confirmed (128) cases. § Probable is defined as a single immunoglobulin M (IgM)-positive specimen in late acute phase or convalescent phase of illness. ¶ Laboratory-confirmed results were positive by polymerase chain reaction (PCR) or viral isolation. ** If not specified otherwise, the number of cases from each country is one. Unknown indicates that no location-specific travel history was provided. Does not include travel destinations of persons with probable cases. †† Of the 518 probable cases reported to ArboNET, 50 probable cases also were reported to CDCDB. These 50 probable cases were subtracted from the ArboNET total and included in the CDCDB total, resulting in 468 probable cases. §§ Of 135 laboratory-confirmed cases reported to ArboNET, seven laboratory-confirmed cases also were reported to CDCDB. These seven cases were subtracted from the ArboNET total and included in the CDCDB total, resulting in 128 laboratory-confirmed cases. |
|||||||||
FIGURE. Number and incidence of laboratory-confirmed cases* per 100 million U.S. travelers† --- combined ArboNET and CDC Dengue Branch (CDCDB), 1996--2008
* Based on 1996--2005 data from CDCDB, and 2006--2008 data from the CDCDB and ArboNET electronic surveillance system.
† Source: Office of Travel and Tourism Industries. 2007 United States resident travel abroad. Washington, DC: US Department of Commerce, International Trade Administration, Office of Travel and Tourism Industries; 2008. Available at http://www.tinet.ita.doc.gov/outreachpages/download_data_table/2007_us_travel_abroad.pdf.
Alternate Text: The figure above shows the number and incidence of laboratory-confirmed cases per 100 million U.S. travelers from combined data from ArboNET and CDC Dengue Branch (CDCDB), from 1996-2008. During 2006-2008, an average of 244 confirmed and probable travel-associated dengue cases were identified by ArboNET or CDCDB annually, compared with an annual average of 33.5 cases (range: 13-77 cases) identified during 1990-2005.
|
After the Council of State and Territorial Epidemiologists (CSTE) recommended addition of dengue to the list of nationally notifiable diseases, in January 2010, CDC added dengue to the list. Public health jurisdictions are encouraged to report cases via the ArboNET system. Health-care providers are encouraged to submit specimens to the CDC Dengue Branch for diagnostic testing, as follows: • Obtain an acute phase (0--5 days after onset of symptoms) serum sample, for directly detecting dengue virus. • Obtain a convalescent phase serum sample, preferably 1--2 weeks after the first sample, for detecting antidengue antibody. Serologic testing can detect diagnostic levels of antidengue immunoglobulin M antibody for approximately 30 days after symptom onset and longer in some patients. • To obtain viral identification and serologic diagnosis, send specimens through state or territorial health departments to the Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, 1324 Calle Cañada, San Juan, PR 00920-3860; telephone 787-706-2399; fax 787-706-2496. • Attach a summary of clinical and epidemiologic information to all serum samples; be sure to include date of disease onset, date of sample collection, and detailed recent travel history. Additional information is available at http://www.cdc.gov/dengue/resources/testpoleng_2.pdf. |
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
 ShareCompartir
ShareCompartir


