Notes from the Field: An Outbreak of Shiga Toxin–Producing Escherichia coli O121 Infections Associated with Flour — Canada, 2016–2017
Weekly / July 7, 2017 / 66(26);705–706
Vanessa Morton, MSc1; Joyce M. Cheng, MPH1; Davendra Sharma, MSc2; Ashley Kearney, MSc3 (View author affiliations)
View suggested citationOn December 29, 2016, PulseNet Canada identified a cluster of six Escherichia coli non-O157 isolates with a matching pulsed-field gel electrophoresis (PFGE) pattern combination that was new to the PulseNet Canada database. The patients resided in three geographically distinct provinces. In January 2017, the Public Health Agency of Canada (PHAC) initiated an investigation with local, provincial, and federal partners to investigate the source of the outbreak.
A case was defined as isolation of E. coli non-O157 with the outbreak PFGE pattern or closely related by whole genome sequencing (WGS) in a Canadian resident or visitor with onset of symptoms of gastroenteritis on or after November 1, 2016. Patients’ illness onset dates ranged from November 2016 to April 2017 (Figure). As of May 23, 2017, a total of 29 cases were identified in six provinces (Alberta, British Columbia, Newfoundland and Labrador, Ontario, Quebec, and Saskatchewan). One additional case was identified in a U.S. resident who traveled to Canada during the exposure period. Patients’ ages ranged from 2–79 years (median = 23.5 years) and 50% were female. Eight patients were hospitalized, and one developed hemolytic uremic syndrome. Clinical isolates were typed as E. coli O121:H19 (one case was typed as E. coli O121:H undetermined) with Shiga toxin 2–producing genes by in silico toxin testing and had closely related PFGE patterns and WGS.
Initial investigation into the source of the outbreak did not identify any clear hypotheses; common exposures were ground beef, sausage style deli-meats, pizza, and pork, but the data did not converge on any specific products. Patients were reinterviewed by PHAC using an open-ended approach. Knowledge of a recent E. coli O121 flour-associated outbreak prompted interviewers to ask about baking and exposure to raw flour or dough (1). Patients were also asked if any food items of interest, including flour, were available for testing.
In March 2017, E. coli O121 with the outbreak PFGE pattern was isolated from an open flour sample from a patient’s home and a closed sample collected at a retail store, both of the same brand and production date. The clinical and flour isolates grouped together, with only 0–6 whole genome multilocus sequence typing allele differences. As a result of these findings, a product recall was issued. Based on possible connections to the recalled lot of flour, market sampling of flour within certain periods was initiated. The investigation led to additional recalls of flour and many secondary products (2).
As of May 23, 2017, 22 patients had been asked about flour exposure in the 7 days before illness onset; 16 (73%) reported that the implicated brand of flour was used or probably used in the home during the exposure period. Comparison data on the expected proportion with exposure to this brand of flour were not available. Eleven of these sixteen patients reported they ate or probably ate raw dough during their exposure period.
This is the first national outbreak of non-O157 Shiga toxin–producing E. coli infections identified in Canada and the first Canadian outbreak linked to flour. An open-ended interview approach and flour sampling were used to implicate flour as the source. Because of the recent emergence of E. coli outbreaks linked to flour, public health professionals should consider flour as a possible source in E. coli outbreaks and communicate the risk associated with exposure to flour, raw batter, and dough in public health messaging.
Acknowledgments
Health Canada; British Columbia Centre for Disease Control; British Columbia Centre for Disease Control Public Health Laboratory; Alberta Health; Alberta Health Services; Alberta Agriculture and Forestry; Saskatchewan Ministry of Health; Public Health Ontario; Ontario Ministry of Health and Long-Term Care; Ministère de la Santé et des Services sociaux du Québec; the Newfoundland and Labrador Regional Health Authorities and Department of Health and Community Services; CDC; Washington State Department of Health; local and regional health authorities; Service Newfoundland and Labrador.
Conflict of Interest
No conflicts of interest were reported.
Corresponding author: Joyce Cheng, joyce.cheng@phac-aspc.gc.ca, 519-826-2494.
1Centre for Foodborne, Environmental, and Zoonotic Infectious Diseases, Public Health Agency of Canada; 2Office of Food Safety and Recall, Canadian Food Inspection Agency; 3National Microbiology Laboratory, Public Health Agency of Canada.
References
- CDC. Multistate outbreak of Shiga toxin-producing Escherichia coli infections linked to flour (Final Update). Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://www.cdc.gov/ecoli/2016/o121-06-16/
- Canadian Food Inspection Agency. Canadian Food Inspection Agency’s (CFIA) investigation into E. coli O121 in flour and flour products. Mississauga, Canada: Canadian Food Inspection Agency; 2017. http://www.inspection.gc.ca/food/information-for-consumers/food-safety-investigations/e-coli-o121/eng/1492621159359/1492621214587
 FIGURE. Number of confirmed cases of Escherichia coli O121 infection (n = 30),* by week of symptom onset — Canada, November 2016–April 2017
FIGURE. Number of confirmed cases of Escherichia coli O121 infection (n = 30),* by week of symptom onset — Canada, November 2016–April 2017
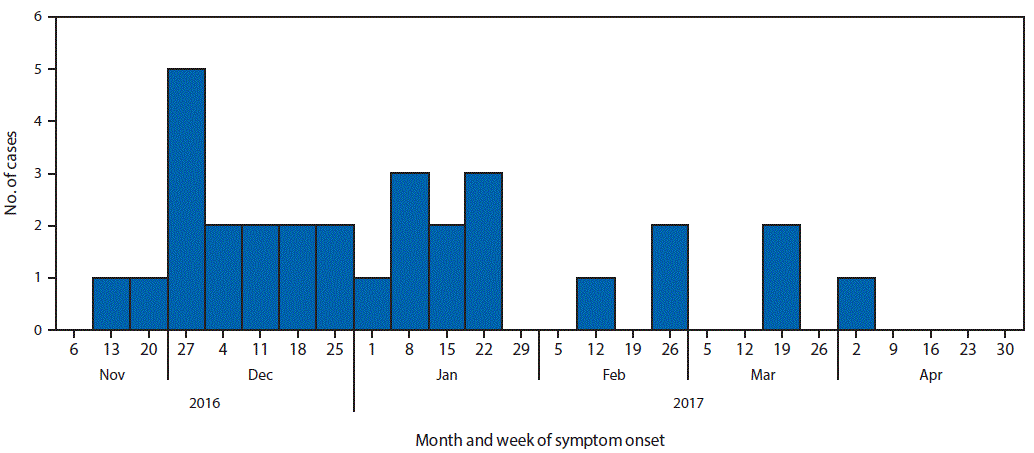
* One case occurred in a U.S. resident who traveled to Canada during the exposure period.
The figure above is a histogram showing the number of confirmed cases of Escherichia coli O121 infection, by week of symptom onset in Canada during November 2016–April 2017.
Suggested citation for this article: Morton V, Cheng JM, Sharma D, Kearney A. Notes from the Field: An Outbreak of Shiga Toxin–Producing Escherichia coli O121 Infections Associated with Flour — Canada, 2016–2017. MMWR Morb Mortal Wkly Rep 2017;66:705–706. DOI: http://dx.doi.org/10.15585/mmwr.mm6626a6.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: July 5, 2017
- Page last updated: July 5, 2017
- Content source:


 ShareCompartir
ShareCompartir