Canakinumab
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | IL-1β |
| Names | |
| Trade names | Ilaris |
| Other names | ACZ885, ACZ-885 |
| Clinical data | |
| Main uses | Periodic fever syndromes, gout, Still's disease[2] |
| Side effects | Infections of the nose and throat, abdominal pain, nausea, injection-site reactions[2][3] |
| Pregnancy category |
|
| Routes of use | Intravenous, subcutaneous |
| External links | |
| AHFS/Drugs.com | Monograph |
| US NLM | Canakinumab |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C6452H9958N1722O2010S42 |
| Molar mass | 145200 g·mol−1 |
Canakinumab, sold under the brand name Ilaris, is a medication used to treat periodic fever syndromes, gout, and Still's disease.[2] The periodic fever syndromes it is used in include cryopyrin-associated periodic syndromes (CAPS), familial mediterranean fever (FMF), tumour necrosis factor receptor associated periodic syndrome (TRAPS), and HIDS/MKD.[2] It is given by injection under the skin.[2]
Common side effects include infections of the nose and throat, abdominal pain, nausea, and injection-site reactions.[2][3] Other side effects may include infections.[3] Those on the medication should not receive live vaccines.[3] It is a monoclonal antibody that binds to and blocks interleukin-1 beta. [2]
Canakinumab was approved for medical use in the United States and Europe in 2009.[4][2] In the United Kingdom 150 mg costs the NHS about £9,900 as of 2021.[5] This amount in the United States is about 17,500 USD.[6]
Medical uses
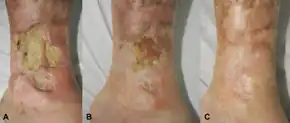
Canakinumab was approved for the treatment of cryopyrin-associated periodic syndromes (CAPS) by the U.S. Food and Drug Administration (FDA) in June 2009[8] and by the European Medicines Agency (EMA) in October 2009.[9][10] CAPS is a spectrum of autoinflammatory syndromes including Familial Cold Autoinflammatory Syndrome (FCAS), Muckle–Wells syndrome (MWS), and Neonatal-Onset Multisystem Inflammatory Disease (NOMID).
In September 2016, the FDA approved the use of canakinumab for three additional rare and serious auto-inflammatory diseases:[11] tumor necrosis factor receptor associated periodic syndrome (TRAPS), hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD), and familial mediterranean fever (FMF).[11]
In June 2020, canakinumab was approved in the United States for the indication to treat active Still's disease, including adult-onset Still's disease (AOSD).[12]
In the European Union, canakinumab is indicated for autoinflammatory periodic fever syndromes, cryopyrin-associated periodic syndromes (CAPS), tumour necrosis factor receptor associated periodic syndrome (TRAPS), hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD), familial Mediterranean fever (FMF), Still's disease, and gouty arthritis.[9]
Dosage
It is used at a dose of 150 to 300 mg every 4 weeks.[3]
Side effects
The FDA prescribing information for canakinumab (Ilaris) includes a warning for potential increased risk of serious infections due to IL-1 blockade.[12] Macrophage activation syndrome (MAS) is a known, life-threatening disorder that may develop in people with rheumatic conditions, in particular Still's disease, and should be aggressively treated.[12] Treatment with immunosuppressants may increase the risk of malignancies.[12] People are advised not to receive live vaccinations during treatment.[12][3]
Mechanism of action
It has no cross-reactivity with other members of the interleukin-1 family, including interleukin-1 alpha.[13]
History
Canakinumab was being developed by Novartis for the treatment of rheumatoid arthritis, but this trial was completed in October 2009.[14] Canakinumab is also in phase I clinical trials as a possible treatment for chronic obstructive pulmonary disease,[15] gout, and coronary artery disease (the CANTOS trial[16]). It is also in trials for schizophrenia.[17] In gout, it may result in better outcomes than a low dose of a steroid, but costs five thousand times more.[18] One 150 mg subcutaneous injection, usually needed every two weeks, costs over $16,700.
On August 27, 2017, the results of the CANTOS trial were announced at the European Society of Cardiology and published in The Lancet and The New England Journal of Medicine.[19] Those treated in CANTOS had a 15% reduction in deaths from heart attacks, stroke and cardiovascular disease combined. However, there were serious side-effects and no statistically significant overall survival benefit. Although the CANTOS study says, "Overall, canakinumab was tolerated well with essentially identical discontinuation rates compared to placebo. Mild neutropenia and thrombocytopenia were slightly more common in those treated with canakinumab. Rates of death due to infection or sepsis were low but more likely in the canakinumab group compared to placebo (incidence rate 0.31 vs. 0.18 per 100 person-years, P = 0.02). In terms of the types of infections that occurred during follow up, only pseudomembranous colitis was more common in the canakinumab group; no evidence of opportunistic infection was observed, data emphasizing that canakinumab is not a clinically immunosuppressive intervention. Further demonstrating this issue, random allocation to canakinumab as compared to placebo in CANTOS resulted in large and highly significant dose-dependent reductions in cancer fatality, incident lung cancer, and fatal lung cancer."[20] Nonetheless, David Goff, director of the division of cardiovascular sciences at the National Heart, Lung and Blood Institute feels the "public health impact potential is really substantial," and estimates that in the United States 3 million people might benefit from canakinumab.[19] Further analysis on data from the CANTOS trial also showed a significant reduction in lung cancer incidence and mortality in the canakinumab treated group compared to placebo.
References
- ↑ Rondeau JM, Ramage P, Zurini M, Gram H (2015). "The molecular mode of action and species specificity of canakinumab, a human monoclonal antibody neutralizing IL-1β". mAbs. 7 (6): 1151–60. doi:10.1080/19420862.2015.1081323. PMC 4966334. PMID 26284424.
- 1 2 3 4 5 6 7 8 "Ilaris". Archived from the original on 11 June 2020. Retrieved 29 December 2021.
- 1 2 3 4 5 6 "Ilaris- canakinumab injection, powder, lyophilized, for solution Ilaris- canakinumab injection, solution". DailyMed. 14 September 2019. Archived from the original on 4 August 2020. Retrieved 16 June 2020.
- ↑ "Canakinumab Monograph for Professionals". Drugs.com. Archived from the original on 4 July 2018. Retrieved 29 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 890. ISBN 978-0857114105.
- ↑ "Ilaris Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 27 January 2021. Retrieved 29 December 2021.
- ↑ Efthimiou, Petros (4 January 2019). Auto-Inflammatory Syndromes: Pathophysiology, Diagnosis, and Management. Springer. p. 54. ISBN 978-3-319-96929-9. Archived from the original on 30 January 2022. Retrieved 29 January 2022.
- ↑ "New biological therapy Ilaris approved in US to treat children and adults with CAPS, a serious life-long auto-inflammatory disease" (Press release). Novartis. 18 June 2009. Archived from the original on 22 June 2009. Retrieved 28 July 2009.
- 1 2 "Ilaris EPAR". European Medicines Agency (EMA). Archived from the original on 11 June 2020. Retrieved 16 June 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Wan Y (29 October 2009). "Canakinumab (Ilaris) and rilonacept (Arcalyst) approved in EU for treatment of cryopyrin-associated periodic syndrome". National electronic Library for Medicines. Archived from the original on 2 October 2011. Retrieved 14 April 2010.
- 1 2 "FDA approves expanded indications for Ilaris for three rare diseases" (Press release). U.S. Food and Drug Administration (FDA). 23 September 2016. Archived from the original on 25 February 2021. Retrieved 3 October 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 "FDA Approves First Treatment for Adult Onset Still's Disease, a Severe and Rare Disease". U.S. Food and Drug Administration (FDA) (Press release). 16 June 2020. Archived from the original on 17 June 2020. Retrieved 16 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. (June 2009). "Use of canakinumab in the cryopyrin-associated periodic syndrome". The New England Journal of Medicine. 360 (23): 2416–25. doi:10.1056/NEJMoa0810787. PMID 19494217.
- ↑ Clinical trial number NCT00784628 for "Safety, Tolerability and Efficacy of ACZ885 (Canakinumab) in Patients With Active Rheumatoid Arthritis" at ClinicalTrials.gov
- ↑ Yasothan U, Kar S (2008). "Therapies for COPD". Nat Rev Drug Discov. 7 (4): 285. doi:10.1038/nrd2533. S2CID 29625221.
- ↑ "CANTOS Summary". theCANTOS.org. Archived from the original on 15 October 2017. Retrieved 6 June 2017.
- ↑ "Canakinumab Add-On Treatment for Schizophrenia (CATS) Study". NeuRA. Archived from the original on 4 November 2016. Retrieved 4 November 2016.
- ↑ Sivera F, Wechalekar MD, Andrés M, Buchbinder R, Carmona L (September 2014). "Interleukin-1 inhibitors for acute gout". The Cochrane Database of Systematic Reviews. 9 (9): CD009993. doi:10.1002/14651858.CD009993.pub2. PMID 25177840.
- 1 2 Johnson C (27 August 2017). "Major drug study opens up vast new opportunities in combating heart disease". The Washington Post.
- ↑ Aday AW, Ridker PM (2018). "Antiinflammatory Therapy in Clinical Care: The CANTOS Trial and Beyond". Frontiers in Cardiovascular Medicine. 5: 62. doi:10.3389/fcvm.2018.00062. PMC 5996084. PMID 29922680.
External links
| Identifiers: |
|---|
- "Canakinumab". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-06-28. Retrieved 2021-10-03.
- "Canakinumab (heavy chain)". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-05-04. Retrieved 2021-10-03.
- "Canakinumab (light chain)". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-05-04. Retrieved 2021-10-03.
- "Canakinumab". National Cancer Institute. Archived from the original on 2020-07-14. Retrieved 2021-10-03.