Deracoxib
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Deramaxx |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | High (more than 90%) |
| Metabolism | Hepatic biotransformation |
| Elimination half-life | 3 hours at 2–3 mg/kg |
| Excretion | In feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.234.875 |
| Chemical and physical data | |
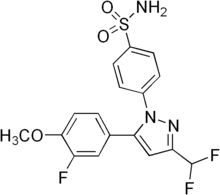
| Formula | C17H14F3N3O3S |
| Molar mass | 397.37 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Deracoxib (trade name Deramaxx) is a nonsteroidal anti-inflammatory drug (NSAID) of the coxib class, used in dogs to treat pain associated with osteoarthritis, or to prevent pain following orthopedic or dental surgery. It is available as beef-flavored tablets.[1]
Medical uses
Deracoxib is used in dogs for the control of pain and inflammation associated with osteoarthritis and for the prevention of pain following orthopedic surgery or dental procedures.[2]
In cats, the use of deracoxib is not recommended.[3]
Contraindications
Deracoxib is contraindicated for treatment of dogs with hypersensitivity to deracoxib or other NSAIDs, and dogs with gastro-intestinal ulcers, renal disease, hepatic disorders, hypoproteinemia, dehydration, or cardiac disease.
Dogs with renal disease may need dose adjustment (if the benefits of the medication outweigh the risks), while those on concurrent diuretic therapy are at increased risk for NSAID toxicity and should not be given this medication.
The concurrent use of deracoxib with steroids or other NSAIDs should be avoided. The safety of deracoxib has not been established in pregnant or nursing dogs or in dogs younger than 4 months of age.
Adverse effects
The most common adverse effects of treatment with deracoxib are vomiting, anorexia, lethargy and depression.[4] Other adverse effects of deracoxib are caused by its effects on the gastrointestinal tract, and include erosions or ulcerations of the lining of the stomach or intestines.[5]
Serious adverse effects, including ulcers which perforate the gastrointestinal tract, have occurred in dogs administered higher than recommended doses of deracoxib, or dogs administered deracoxib at the same time as (or soon after) other NSAIDs or corticosteroid medications.[4]
Documented adverse side effects include serious and sometimes fatal organ system damage or failure.[6] Other side effects include increase in drinking or urination, jaundice, bloody or black stools, pale gums, hot spots, increased respiration (fast or heavy breathing), incoordination, and behavior changes.
Pharmacology
Deracoxib is a coxib class nonsteroidal anti-inflammatory drug (NSAID).[3] Like other NSAIDs, its effects are caused by inhibition of cyclooxygenase (COX) enzymes.[7] At the doses used to treat dogs, deracoxib causes greater inhibition of COX-2 than of COX-1,[3] but at doses twice those recommended for use in dogs, deracoxib significantly inhibits COX-1 as well.[8]
In dogs, the half-life of deracoxib at the recommended dose is three hours.[8]
Society and culture
In the U.S., deracoxib was first approved for use in dogs in 2002, under the trade name Deramaxx chewable tablets, sold by Novartis Animal Health.[1]
References
- 1 2 "Deramaxx tablets approved by the FDA as the first coxib class drug for veterinary use". dvm360.com. 1 October 2002. Retrieved 2017-08-12.
- ↑ Budsberg SC (2015). "Chapter 8: Nonsteroidal anti-inflammatory drugs". In Gaynor JS, Muir WW (eds.). Handbook of Veterinary Pain Management (3rd ed.). Elsevier Mosby. pp. 142–160. ISBN 9780323222143.
- 1 2 3 Khan SA, McLean MK (March 2012). "Toxicology of frequently encountered nonsteroidal anti-inflammatory drugs in dogs and cats". The Veterinary Clinics of North America. Small Animal Practice. 42 (2): 289–306, vi–vii. doi:10.1016/j.cvsm.2012.01.003. PMID 22381180.
- 1 2 Peterson ME, Talcott PA (2013). "Chapter 65: Nonsteroidal antiinflammatories. Deracoxib". Small animal toxicology (3rd ed.). St. Louis, Mo.: Elsevier. pp. 700–701. ISBN 9781455707171.
- ↑ Papich M (2015). "Deracoxib". Saunders Handbook of Veterinary Drugs: Small and Large Animal (4th ed.). Elsevier Health Sciences. p. 211. ISBN 9780323244862.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2011-04-08. Retrieved 2011-06-23.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Lees P (2013). "Chapter 19: Analgesic, antiinflammatory, antipyretic drugs". In Riviere JE, Papich M (eds.). Veterinary Pharmacology and Therapeutics (9th ed.). John Wiley & Sons. pp. 457–492. ISBN 9781118685907.
- 1 2 Hanson PD, Maddison JE (2008). "Chapter 13: Nonsteroidal anti-inflammatory drugs and chondroprotective agents". In Maddison JE, Page SW, Church DB (eds.). Small animal clinical pharmacology (2nd ed.). Edinburgh: Saunders/Elsevier. pp. 287–308. ISBN 9780702028588.