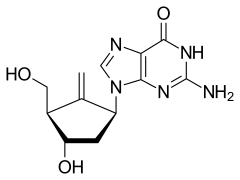
Entecavir
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ɛnˈtɛkəvɪər/ en-TEK-ə-veer |
| Trade names | Baraclude,[1] Entimate, others |
IUPAC name
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 0.5 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| US NLM | Entecavir |
| MedlinePlus | a605028 |
| Legal | |
| License data | |
| Chemical and physical data | |
| Formula | C12H15N5O3 |
| Molar mass | 277.284 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 220 °C (428 °F) value applies to entecavir monohydrate and is a minimum value[3] |
SMILES
| |
InChI
| |
Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection.[1] In those with both HIV/AIDS and HBV antiretroviral medication should also be used.[1] Entecavir is taken by mouth as a tablet or solution.[1]
Common side effects include headache, nausea, high blood sugar, and decreased kidney function.[1] Severe side effects include enlargement of the liver, high blood lactate levels, and liver inflammation if the medication is stopped.[1] While there appears to be no harm from use during pregnancy, this use has not been well studied.[4] Entecavir is in the nucleoside reverse transcriptase inhibitors (NRTIs) family of medications.[1][5] It prevents the hepatitis B virus from multiplying by blocking reverse transcriptase.[1]
Entecavir was approved for medical use in 2005.[1] It is on the World Health Organization's List of Essential Medicines.[6] In the United States, As of 2015, it is not available as a generic medication.[7] The wholesale price is about US$392 for a typical month supply as of 2016 in the United States.[8]
Medical uses
Entecavir is mainly used to treat chronic hepatitis B infection in adults and children 2 years and older with active viral replication and evidence of active disease with elevations in liver enzymes.[9] It is also used to prevent HBV reinfection after liver transplant[10] and to treat HIV patients infected with HBV. Entecavir is weakly active against HIV, but is not recommended for use in HIV-HBV co-infected patients without a fully suppressive anti-HIV regimen[11] as it may select for resistance to lamivudine and emtricitabine in HIV.[12]
The efficacy of entecavir has been studied in several randomized, double-blind, multicentre trials. Entecavir by mouth is effective and generally well tolerated treatment.[13]
Pregnancy and breastfeeding
It is considered pregnancy category C in the United States, and currently no adequate and well-controlled studies exist in pregnant women.[14]
Dosage
The defined daily dose is 0.5 mg (parenteral)[2]
Side effects
The majority of people who use entecavir have little to no side effects.[15] The most common side effects include headache, fatigue, dizziness, and nausea.[9] Less common effects include trouble sleeping and gastrointestinal symptoms such as sour stomach, diarrhea, and vomiting.[16]
Serious side effects from entecavir include lactic acidosis, liver problems, liver enlargement, and fat in the liver.[17]
Laboratory tests may show an increase in alanine transaminase (ALT), hematuria, glycosuria, and an increase in lipase.[18] Periodic monitoring of hepatic function and hematology are recommended.[9]
Mechanism of action
Entecavir is a nucleoside analog,[19] or more specifically, a deoxyguanosine analogue that belongs to a class of carbocyclic nucleosides and inhibits reverse transcription, DNA replication and transcription in the viral replication process. Other nucleoside and nucleotide analogues include lamivudine, telbivudine, adefovir dipivoxil, and tenofovir.
Entecavir reduces the amount of HBV in the blood by reducing its ability to multiply and infect new cells.[20]
Administration
Entecavir is taken by mouth as a tablet or solution. Doses are based on a person's weight.[17] The solution is recommended for children more than 2 years old who weigh up to 30 kg. Entecavir is recommended on an empty stomach at least 2 hours before or after a meal, generally at the same time every day. It is not used in children less than 2 years old. Dose adjustments are also recommended for people with decreased kidney function.[17]
History
- 1992: SQ-34676 at Squibb as part of anti-herpes virus program[21]
- 1997: BMS 200475 developed at BMS pharmaceutical research institute as antiviral nucleoside analogue à Activity demonstrated against HBV, HSV-1, HCMV, VZV in cell lines & no or little activity against HIV or influenza[22]
- Superior activity observed against HBV pushed research towards BMS 200475, its base analogues and its enantiomer against HBV in HepG2.2.15 cell line[22]
- Comparison to other NAs, proven more selective potent inhibitor of HBV by virtue of being Guanine NA[23]
- 1998: Inhibition of hepadnaviral polymerases was demonstrated in vitro in comparison to a number of NAs-TP[24]
- Metabolic studies showed more efficient phosphorylation to triphosphate active form[25]
- 3-year treatment of woodchuck model of CHB à sustained antiviral efficacy and prolonged life spans without detectable emergence of resistance[26]
- Efficacy # LVD resistant HBV replication in vitro[27]
- Superior activity compared to LVD in vivo for both HBeAg+ & HBeAg− patients[28][29]
- Efficacy in LVD refractory CHB patients[30]
- Entecavir was approved by the U.S. FDA in March 2005.
Patent information
Bristol-Myers Squibb was the original patent holder for Baraclude, the brand name of entecavir in the US and Canada. The drug patent expiration for Baraclude was in 2015.[31][32] Entecavir patents were a subject of litigation in the USA between Bristol-Myers-Squibb Co. (the patent owner) and Teva Pharmaceuticals USA (a generic manufacturer). The lawsuit resulted in a relatively rare in the pharmaceutical field patent invalidation for obviousness, which was affirmed on 12 June 2014 by the US Court of Appeals for the Federal Circuit (752 F.32d 967).
On August 26, 2014, Teva Pharmaceuticals USA gained FDA approval for generic equivalents of Baraclude 0.5 mg and 1 mg tablets;[33] Hetero Labs received such approval on August 21, 2015;[34] and Aurobindo Pharma on August 26, 2015.[35]
References
- 1 2 3 4 5 6 7 8 9 "Entecavir". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 28 November 2016.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 30 October 2020. Retrieved 21 September 2020.
- ↑ O'Neil (2006). "The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals". The Merck Index (14th ed.). p. 613. ISBN 978-0-911910-00-1.
- ↑ "Entecavir (Baraclude) Use During Pregnancy". www.drugs.com. Archived from the original on 7 November 2016. Retrieved 6 December 2016.
- ↑ Shetty, Kirti; Wu, George Y. (2009). Chronic Viral Hepatitis: Diagnosis and Therapeutics. Springer Science & Business Media. p. 34. ISBN 9781597455657. Archived from the original on 2018-08-28. Retrieved 2018-05-25.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 76. ISBN 9781284057560.
- ↑ "NADAC as of 2016-11-30 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 30 November 2016. Retrieved 6 December 2016.
- 1 2 3 "Baraclude (entecavir) Tablets for Oral Use & Oral Solution. U.S. Full Prescribing Information. Archived 2014-02-22 at the Wayback Machine" Bristol-Myers Squibb Company, 2005. Revised December 2013.
- ↑ Fung, J; Cheung, C; Chan, SC; et al. (2011). "Entecavir Monotherapy is Effective in Suppressing Hepatitis B Virus After Liver Transplantation". Gastroenterology. 141 (4): 1212–9. doi:10.1053/j.gastro.2011.06.083. PMID 21762659.
- ↑ "Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents" (PDF). Panel on Antiretroviral Guidelines for Adults and Adolescents. Archived (PDF) from the original on 1 November 2016. Retrieved 15 March 2015.
- ↑ McMahon, Moira (21 June 2007). "The Anti-Hepatitis B Drug Entecavir Inhibits HIV-1 Replication and Can Select HIV-1 Variants Resistant to Antiretroviral Drugs". N Engl J Med. 356 (25): 2614–2621. doi:10.1056/NEJMoa067710. PMC 3069686. PMID 17582071.
- ↑ Scott, LJ; Keating, GM (2009). "Entecavir". Drugs. 69 (8): 1003–1033. doi:10.2165/00003495-200969080-00005. PMID 19496629. Archived from the original on 2011-10-08. Retrieved 2010-03-29.
- ↑ "Entecavir (Baraclude) Use During Pregnancy". www.drugs.com. Archived from the original on 2016-11-07. Retrieved 2016-11-07.
- ↑ "Entecavir: Indications, Side Effects, Warnings - Drugs.com". www.drugs.com. Archived from the original on 2016-11-07. Retrieved 2016-11-10.
- ↑ "Entecavir Side Effects in Detail - Drugs.com". www.drugs.com. Archived from the original on 2016-11-10. Retrieved 2016-11-10.
- 1 2 3 "DailyMed - BARACLUDE- entecavir tablet, film coated BARACLUDE- entecavir solution". dailymed.nlm.nih.gov. Archived from the original on 2016-11-08. Retrieved 2016-11-09.
- ↑ "DailyMed - BARACLUDE- entecavir tablet, film coated BARACLUDE- entecavir solution". dailymed.nlm.nih.gov. Archived from the original on 2016-11-09. Retrieved 2016-11-10.
- ↑ Sims KA, Woodland AM (December 2006). "Entecavir: a new nucleoside analog for the treatment of chronic hepatitis B infection". Pharmacotherapy. 26 (12): 1745–57. doi:10.1592/phco.26.12.1745. PMID 17125436.

- ↑ "Entecavir: Indications, Side Effects, Warnings - Drugs.com". www.drugs.com. Archived from the original on 2016-11-07. Retrieved 2016-11-07.
- ↑ Slusarchyk, W. A., A. K. Field, J. A. Greytok, P. Taunk, A. V. Tooumari, M. G. Young, and R. Zahler. 4-Hydroxy-3-(hydroxymethyl)-2-methylcyclopentyl purines and pyrimidines, a novel class of anti-herpesvirus agents. Abstract from the Fifth International Conference on Antiviral Research. Antivir Res 1992.17(Suppl. 1):98
- 1 2 Bisacchi, G. S.; Chao, S. T.; Bachard, C.; Daris, J. P.; Innaimo, S. F.; Jacobs, J. A.; Kocy, O.; Lapointe, P.; Martel, A.; Merchant, Z.; Slusarchyk, W. A.; Sundeen, J. E.; Young, M. G.; Colonno, R.; Zahler, R. (1997). "BMS-200475, a novel carbocyclic 29-deoxyguanosine analog with potent and selective antihepatitis B virus activity in vitro". Bioorg. Med. Chem. Lett. 7 (2): 127–132. doi:10.1016/s0960-894x(96)00594-x.
- ↑ Innaimo, S F; Seifer, M; Bisacchi, G S; Standring, D N; Zahler, R; Colonno, R J (1997). "Identification of BMS-200475 as a Potent and Selective Inhibitor of Hepatitis B Virus. Antimicrob". Agents Chemother. 41 (7): 1444–1448. doi:10.1128/AAC.41.7.1444. PMC 163937. PMID 9210663.
- ↑ Seifer, M.; Hamatake, R. K.; Colonno, R. J.; Standring, D. N. (1998). "In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob". Agents Chemother. 42 (12): 3200–3208. doi:10.1128/AAC.42.12.3200. PMC 106023. PMID 9835515.
- ↑ Yamanaka, G.; Wilson, T.; Innaimo, S.; Bisacchi, G. S.; Egli, P.; Rinehart, J. K.; Zahler, R.; Colonno, R. J. (1999). "Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus. Antimicrob". Agents Chemother. 43: 190–193. doi:10.1128/AAC.43.1.190.
- ↑ Colonno, R. J.; Genovesi, E. V.; Medina, I.; Lamb, L.; Durham, S. K.; Huang, M. L.; Corey, L.; Littlejohn, M.; Locarnini, S.; Tennant, B. C.; Rose, B.; Clark, J. M. (2001). "Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection". J. Infect. Dis. 184 (10): 1236–1245. doi:10.1086/324003. PMID 11679911.
- ↑ Levine, S.; Hernandez, D.; Yamanaka, G.; Zhang, S.; Rose, R.; Weinheimer, S.; Colonno, R. J. (2002). "Efficacies of entecavir against lamivudine-resistant hepatitis B virus replication and recombinant polymerases in vitro. Antimicrob". Agents Chemother. 46 (8): 2525–2532. doi:10.1128/aac.46.8.2525-2532.2002. PMC 127388. PMID 12121928.
- ↑ Chang, T. T. (2006). "A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B.". N. Engl. J. Med. 354 (10): 1001–1010. doi:10.1056/nejmoa051285. PMID 16525137.
- ↑ Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, Colonno R, Fernandes L (9 March 2006). "Entecavir versus Lamivudine for Patients with HBeAg-Negative Chronic Hepatitis B" (PDF). The New England Journal of Medicine. 354 (10): 1011–20. doi:10.1056/NEJMoa051287. PMID 16525138. Archived (PDF) from the original on 25 September 2019. Retrieved 25 September 2019.
- ↑ Sherman, M.; Yurdaydin, C.; Sollano, J.; Silva, M.; Liaw, Y. F.; Cianciara, J.; Boron-Kaczmarska, A.; Martin, P.; Goodman, Z.; Colonno, R. J.; Cross, A.; Denisky, G.; Kreter, B.; Hindes, R. (2006). "Entecavir for the treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B.". Gastroenterology. 130 (7): 2039–2049. doi:10.1053/j.gastro.2006.04.007. PMID 16762627.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Archived from the original on 4 March 2016. Retrieved 2015-08-29.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". Orange Book. Patent and Exclusivity for: N021798. Archived from the original on 15 November 2016. Retrieved 14 November 2016.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Search results from the "OB_Rx" table for query on "202122.". Archived from the original on 22 December 2015. Retrieved 2015-08-29.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Search results from the "OB_Rx" table for query on "205740.". Archived from the original on 4 March 2016. Retrieved 2015-08-29.
- ↑ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Search results from the "OB_Rx" table for query on "206217.". Archived from the original on 4 March 2016. Retrieved 2015-08-29.
External links
| External sites: |
|
|---|---|
| Identifiers: |