Fulvestrant
 | |
| Names | |
|---|---|
| Pronunciation | /fʊlˈvɛstrənt/ fuul-VES-trənt |
| Trade names | Faslodex, others |
| Other names | ICI-182780; ZD-182780; ZD-9238; 7α-[9-[(4,4,5,5,5-Pentafluoropentyl)-sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol |
IUPAC name
| |
| Clinical data | |
| Drug class | Antiestrogen |
| Main uses | Breast cancer[1] |
| Side effects | Nausea, diarrhea, headache, pain, hot flushes, cough, swelling, rash, trouble sleeping[1] |
| Pregnancy category |
|
| Routes of use | Intramuscular injection |
| Typical dose | 500 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Low[3] |
| Protein binding | 99%[3] |
| Metabolism | Hydroxylation, conjugation (glucuronidation, sulfation)[3] |
| Elimination half-life | IM: 40–50 days[3] |
| Chemical and physical data | |
| Formula | C32H47F5O3S |
| Molar mass | 606.78 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Fulvestrant, sold under the brand name Faslodex among others, is a medication used to treat breast cancer.[1] Specifically it is used for hormone receptor (HR) positive cases.[4][5] It is given by injection into a muscle.[1]
Common side effects include nausea, diarrhea, headache, pain, hot flushes, cough, swelling, rash, and trouble sleeping.[1] Other side effects may include low white blood cells, low red blood cells, low platelets, infection, and liver problems.[5] It should not be used in pregnancy or breastfeeding.[5] It is a selective estrogen receptor degrader (SERD) which binds to estrogen receptor resulting in their destruction.[6]
Fulvestrant was approved for medical use in the United States in 2002 and Europe in 2004.[1][5] It is available as a generic medication.[2] In the United Kingdom it costs the NHS about £520 per dose of 2021.[2] This amount in the United States costs about 100 USD.[7]
Medical uses
Breast cancer
Fulvestrant is used for the treatment of hormone receptor positive metastatic breast cancer or locally advanced unresectable disease in postmenopausal women; it is given by injection.[8] A 2017 Cochrane review found it is as safe and effective as first line or second line endocrine therapy.[8]
It is also used to treat ER-positive, HER2-negative advanced or metastatic breast cancer in combination with palbociclib in women with disease progression after first-line endocrine therapy.[9] Due to the medication's having a chemical structure similar to that of estrogen, it can interact with immunoassays for blood estradiol concentrations and show falsely elevated results.[10][11][12] This can improperly lead to discontinuing the treatment.[10]
Early puberty
Fulvestrant has been used in the treatment of peripheral precocious puberty in girls with McCune–Albright syndrome.[13][14][15]
Dosage
For breast cancer it is generally given at a dose of 500 mg every two weeks for three doses, and than 500 mg a month.[5]
Fulvestrant is provided in a castor oil solution also containing alcohol, benzyl alcohol, and benzyl benzoate.[9] It is supplied at a concentration of 250 mg/5 mg.[9]
Contraindications
Fulvestrant should not be used in women with kidney failure or who are pregnant.[9][16]
Side effects
Very common (occurring in more than 10% of people) adverse effects include nausea, injection site reactions, weakness, and elevated transaminases. Common (between 1% and 10%) adverse effects include urinary tract infections, hypersensitivity reactions, loss of appetite, headache, blood clots in veins, hot flushes, vomiting, diarrhea, elevated bilirubin, rashes, and back pain.[16] In a large clinical trial, the incidence of venous thromboembolism (VTE) with fulvestrant was 0.9%.[9]
Pharmacology
Pharmacodynamics
Fulvestrant is an antiestrogen which acts as an antagonist of the estrogen receptor (ER) and additionally as a selective estrogen receptor degrader (SERD).[6] It works by binding to the estrogen receptor and making it more hydrophobic, which makes the receptor unstable and misfold, which in turn leads normal processes inside the cell to degrade it.[6]
In addition to its antiestrogenic activity, fulvestrant is an agonist of the G protein-coupled estrogen receptor (GPER), albeit with relatively low affinity (10–100 nM, relative to 3–6 nM for estradiol).[17][18][19][20][21]
Pharmacokinetics
Fulvestrant after an intramuscular injection is slowly absorbed and maximal levels (Cmax) are reached after 5 days on average with a range of 2 to 19 days.[22] The elimination half-life of fulvestrant with intramuscular injection is 40 to 50 days.[23][9] This is 40 times longer than the half-life of fulvestrant by intravenous injection, indicating that its long half-life with intramuscular injection is due to slow absorption from the injection site.[22] Levels of fulvestrant with 500 mg/month by intramuscular injection (and a single additional 500 mg loading dose on day 15 of therapy) in postmenopausal women with advanced breast cancer were 25.1 ng/mL (25,100 pg/mL) at peak and 28.0 ng/mL (28,000 pg/mL) at trough with a single dose and 28.0 ng/mL (28,000 pg/mL) at peak and 12.2 ng/mL (12,200 pg/mL) at trough after multiple doses at steady state.[9]
Fulvestrant does not cross the blood–brain barrier in animals and may not in humans as well.[24][25][26] Accordingly, no effects of fulvestrant on brain function have been observed in preclinical or clinical research.[25][26] Fulvestrant is highly (99%) bound to plasma proteins.[23][9] It is bound to very low density lipoprotein, low density lipoprotein, and high density lipoprotein, but not to sex hormone-binding globulin.[23]
Fulvestrant appears to be metabolized along similar pathways as endogenous steroids; CYP3A4 may be involved, but non-cytochrome P450 routes appear to be more important. It does not inhibit any cytochrome P450 enzymes. Elimination is almost all via feces.[16]
Fulvestrant can form colloidal aggregates at certain concentration ranges and this can limit its activity as well as produce bell-shaped concentration–response curves.[27][28][29]
Chemistry
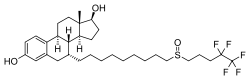
Fulvestrant, also known as 7α-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estradiol, is a synthetic estrane steroid and a derivative of estradiol. An alkyl-sulfinyl moiety was added to the endogenous estrogen receptor ligand.[6]
It was discovered through rational drug design, but was selected for further development via phenotypic screening.[30]
History
Fulvestrant was the first selective estrogen receptor degrader to be approved.[6] It was approved in the United States in 2002[9] and in Europe in 2004.[16]
Society and culture
NICE evaluation
The U.K. National Institute for Health and Clinical Excellence (NICE) said in 2011 that it found no evidence Faslodex was significantly better than existing treatments, so its widespread use would not be a good use of resources for the country's National Health Service. The first month's treatment of Faslodex, which starts with a loading dose, costs £1,044.82 ($1,666), and subsequent treatments cost £522.41 a month. In the 12 months ending June 2015, the UK price (excluding VAT) of a month's supply of anastrozole (Arimidex), which is off patent, cost 89 pence/day, and letrozole (Femara) cost £1.40/day.[31][32][33]
Patent extension
The original patent for Faslodex expired in October 2004. Drugs subject to pre-marketing regulatory review are eligible for patent extension, and for this reason AstraZeneca got an extension of the patent to December 2011.[34][35] AstraZeneca has filed later patents. A generic version of Faslodex has been approved by the FDA. However, this does not mean that the product will necessarily be commercially available - possibly because of drug patents and/or drug exclusivity.[36] A later patent for Faslodex expires in January 2021.[37] Atossa Genetics has a patent for the administration of fulvestrant into the breast via a microcatheter invented by Susan Love.[38]
Research
Fulvestrant was studied in endometrial cancer but results were not promising and as of 2016 development for this use was abandoned.[39]
Because fulvestrant cannot be given orally, efforts have been made to develop SERD drugs that can be taken by mouth, including brilanestrant and elacestrant.[6] The clinical success of fulvestrant also led to efforts to discover and develop a parallel drug class of selective androgen receptor degraders (SARDs).[6]
ZB716, or fulvestrant-3-boronic acid, is an oral prodrug of fulvestrant which is under development.[40][41][42]
References
- 1 2 3 4 5 6 "Fulvestrant". The American Society of Health-System Pharmacists. Archived from the original on 2 February 2017. Retrieved 8 January 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 996. ISBN 978-0857114105.
- 1 2 3 4 Dörwald FZ (4 February 2013). Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds. John Wiley & Sons. pp. 486–. ISBN 978-3-527-64565-7. Archived from the original on 10 October 2021. Retrieved 19 September 2021.
- ↑ "DailyMed - FULVESTRANT injection, solution". dailymed.nlm.nih.gov. Archived from the original on 12 December 2021. Retrieved 12 December 2021.
- 1 2 3 4 5 "Faslodex". Archived from the original on 19 October 2021. Retrieved 12 December 2021.
- 1 2 3 4 5 6 7 Lai AC, Crews CM (February 2017). "Induced protein degradation: an emerging drug discovery paradigm". Nature Reviews. Drug Discovery. 16 (2): 101–114. doi:10.1038/nrd.2016.211. PMC 5684876. PMID 27885283.
- ↑ "Faslodex Prices and Faslodex Coupons - GoodRx". GoodRx. Retrieved 12 December 2021.
- 1 2 Lee CI, Goodwin A, Wilcken N (January 2017). "Fulvestrant for hormone-sensitive metastatic breast cancer". The Cochrane Database of Systematic Reviews. 1: CD011093. doi:10.1002/14651858.CD011093.pub2. PMC 6464820. PMID 28043088.
- 1 2 3 4 5 6 7 8 9 "US Label: Fulvestrant" (PDF). FDA. July 2016. Archived (PDF) from the original on 2021-10-10. Retrieved 2021-09-19.
- 1 2 "Estradiol immunoassays – interference from the drug fulvestrant (Faslodex®) may cause falsely elevated estradiol results Medical safety alert - GOV.UK". UK Medicines and Healthcare products Regulatory Agency. 24 March 2016. Archived from the original on 19 July 2017. Retrieved 19 September 2021.
- ↑ Owen LJ, Monaghan PJ, Armstrong A, Keevil BG, Higham C, Salih Z, Howell S (February 2019). "Oestradiol measurement during fulvestrant treatment for breast cancer". Br J Cancer. 120 (4): 404–406. doi:10.1038/s41416-019-0378-9. PMC 6461991. PMID 30679781.
- ↑ Samuel E, Chiang C, Jennens R, Faulkner D, Francis PA (February 2020). "Fulvestrant falsely elevates oestradiol levels in immunoassays in postmenopausal women with breast cancer". Eur J Cancer. 126: 104–105. doi:10.1016/j.ejca.2019.10.015. PMID 31927211. S2CID 210166996.
- ↑ Fuqua JS (June 2013). "Treatment and outcomes of precocious puberty: an update". The Journal of Clinical Endocrinology and Metabolism. 98 (6): 2198–207. doi:10.1210/jc.2013-1024. PMID 23515450.
- ↑ Zacharin M (May 2019). "Disorders of Puberty: Pharmacotherapeutic Strategies for Management". Handbook of Experimental Pharmacology. 261: 507–538. doi:10.1007/164_2019_208. ISBN 978-3-030-50493-9. PMID 31144045.
- ↑ Sims EK, Garnett S, Guzman F, Paris F, Sultan C, Eugster EA (September 2012). "Fulvestrant treatment of precocious puberty in girls with McCune-Albright syndrome". International Journal of Pediatric Endocrinology. 2012 (1): 26. doi:10.1186/1687-9856-2012-26. PMC 3488024. PMID 22999294.
- 1 2 3 4 "Faslodex 250 mg solution for injection - Summary of Product Characteristics". UK Electronic Medicines Compendium. 21 July 2016. Archived from the original on 10 October 2021. Retrieved 19 September 2021.
- ↑ Prossnitz ER, Arterburn JB (July 2015). "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacol. Rev. 67 (3): 505–40. doi:10.1124/pr.114.009712. PMC 4485017. PMID 26023144.
- ↑ Thomas P, Pang Y, Filardo EJ, Dong J (February 2005). "Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells". Endocrinology. 146 (2): 624–32. doi:10.1210/en.2004-1064. PMID 15539556.
- ↑ Prossnitz ER, Barton M (August 2011). "The G-protein-coupled estrogen receptor GPER in health and disease". Nat Rev Endocrinol. 7 (12): 715–26. doi:10.1038/nrendo.2011.122. PMC 3474542. PMID 21844907.
- ↑ Prossnitz ER, Barton M (May 2014). "Estrogen biology: new insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ↑ Barton M (August 2012). "Position paper: The membrane estrogen receptor GPER--Clues and questions". Steroids. 77 (10): 935–42. doi:10.1016/j.steroids.2012.04.001. PMID 22521564. S2CID 35909008.
- 1 2 Robertson JF, Harrison M (March 2004). "Fulvestrant: pharmacokinetics and pharmacology". Br J Cancer. 90 Suppl 1: S7–10. doi:10.1038/sj.bjc.6601630. PMC 2750771. PMID 15094758.
- 1 2 3 Croxtall JD, McKeage K (February 2011). "Fulvestrant: a review of its use in the management of hormone receptor-positive metastatic breast cancer in postmenopausal women". Drugs. 71 (3): 363–80. doi:10.2165/11204810-000000000-00000. PMID 21319872.
- ↑ Robertson JF (November 2001). "ICI 182,780 (Fulvestrant)--the first oestrogen receptor down-regulator--current clinical data". Br. J. Cancer. 85 Suppl 2: 11–4. doi:10.1054/bjoc.2001.1982 (inactive 31 May 2021). PMC 2375169. PMID 11900210.
{{cite journal}}: CS1 maint: DOI inactive as of May 2021 (link) - 1 2 Howell A, Abram P (2005). "Clinical development of fulvestrant ("Faslodex")". Cancer Treat. Rev. 31 Suppl 2: S3–9. doi:10.1016/j.ctrv.2005.08.010. PMID 16198055.
- 1 2 Bundred N, Howell A (April 2002). "Fulvestrant (Faslodex): current status in the therapy of breast cancer". Expert Rev Anticancer Ther. 2 (2): 151–60. doi:10.1586/14737140.2.2.151. PMID 12113237. S2CID 20294814.
- ↑ Ganesh AN, Donders EN, Shoichet BK, Shoichet MS (April 2018). "Colloidal aggregation: from screening nuisance to formulation nuance". Nano Today. 19: 188–200. doi:10.1016/j.nantod.2018.02.011. PMC 6150470. PMID 30250495.
- ↑ Ganesh AN, Aman A, Logie J, Barthel BL, Cogan P, Al-Awar R, Koch TH, Shoichet BK, Shoichet MS (April 2019). "Colloidal Drug Aggregate Stability in High Serum Conditions and Pharmacokinetic Consequence". ACS Chem Biol. 14 (4): 751–757. doi:10.1021/acschembio.9b00032. PMC 6474797. PMID 30840432.
- ↑ Owen SC, Doak AK, Ganesh AN, Nedyalkova L, McLaughlin CK, Shoichet BK, Shoichet MS (March 2014). "Colloidal drug formulations can explain "bell-shaped" concentration-response curves". ACS Chem Biol. 9 (3): 777–84. doi:10.1021/cb4007584. PMC 3985758. PMID 24397822.
- ↑ Moffat JG, Rudolph J, Bailey D (August 2014). "Phenotypic screening in cancer drug discovery - past, present and future". Nature Reviews. Drug Discovery. 13 (8): 588–602. doi:10.1038/nrd4366. PMID 25033736. S2CID 5964541.
- ↑ UK Department of Health Commercial Medicines Unit Electronic Medicines Information Tool Archived 2021-08-17 at the Wayback Machine, London, 2015
- ↑ UK’s NICE says no to AstraZeneca breast cancer drug Faslodex Archived 2012-04-26 at the Wayback Machine, The Pharma Letter, 10 November 2011
- ↑ National Institute for Health and Clinical Excellence Guidance Archived 2011-04-03 at the Wayback Machine Breast cancer (metastatic) - fulvestrant
- ↑ Patent Term Extensions Archived 2015-01-08 at the Wayback Machine The United States Patent and Trademark Office.
- ↑ Determination of Regulatory Review Period for Purposes of Patent Extension; FASLODEX Archived 2021-11-01 at the Wayback Machine A Notice by the Food and Drug Administration on 04/17/2003
- ↑ Generic Faslodex Availability Archived 2017-07-31 at the Wayback Machine, Drugs.COM
- ↑ Pink Ribbon Blues: How Breast Cancer Culture Undermines Women's Health Archived 2013-06-07 at the Wayback Machine By Gayle A. Sulik, Oxford University Press (Oct. 2010)
- ↑ US granted 6638727, Hung DT, Love S, "Methods for identifying treating or monitoring asymptomatic patients for risk reduction or therapeutic treatment of breast cancer", issued 28 October 2003, assigned to Cytyc Health Corp
- ↑ Battista MJ, Schmidt M (2016). "Fulvestrant for the treatment of endometrial cancer". Expert Opinion on Investigational Drugs. 25 (4): 475–83. doi:10.1517/13543784.2016.1154532. PMID 26882357. S2CID 207477738.
- ↑ Ahmad, I., Mathew, S., & Rahman, S. (2020). Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer. RSC Medicinal Chemistry, 11(4), 438–454. https://doi.org/10.1039/C9MD00570F
- ↑ Liu J, Zheng S, Akerstrom VL, Yuan C, Ma Y, Zhong Q, Zhang C, Zhang Q, Guo S, Ma P, Skripnikova EV, Bratton MR, Pannuti A, Miele L, Wiese TE, Wang G (2016). "Fulvestrant-3 Boronic Acid (ZB716): An Orally Bioavailable Selective Estrogen Receptor Downregulator (SERD)". J. Med. Chem. 59 (17): 8134–40. doi:10.1021/acs.jmedchem.6b00753. PMC 5499704. PMID 27529700.
- ↑ "ClinicalTrials.gov: NCT04669587". Archived from the original on 2021-11-01. Retrieved 2021-09-19.
External links
| External sites: |
|
|---|---|
| Identifiers: |