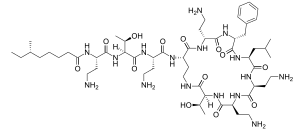
Polymyxin B
 | |
 | |
| Names | |
|---|---|
| Trade names | Poly-Rx, others |
IUPAC name
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | Topical, intramuscular, intravenous, intrathecal, eye drops |
| Defined daily dose | 150 mg (by injection)[2] 3 million units (by mouth)[3] |
| External links | |
| AHFS/Drugs.com | Systemic: Monograph Eye and ear: Monograph |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C56H100N16O17S |
| Molar mass | 1301.57 g·mol−1 |
Polymyxin B, sold under the brand name Poly-Rx among others, is an antibiotic used to treat meningitis, pneumonia, sepsis, and urinary tract infections.[1] While it is useful for many Gram negative infections, it is not useful for Gram positive infections.[1] It can be given by injection into a vein, muscle, or cerebrospinal fluid or inhaled.[1] The injectable form is generally only used if other options are not available.[4] It is also available as the combinations bacitracin/polymyxin B and neomycin/polymyxin B/bacitracin for use on the skin.[5][6]
Common side effects when given by injection include kidney problems, neurological problems, fever, itchiness, and rash.[1] Injections into muscle may result in significant pain.[1] Other serious side effects may include fungal infections, anaphylaxis, and muscle weakness.[1] It is unclear if use during pregnancy is safe for the baby.[1] Polymyxin B works by breaking down the cytoplasmic membrane which generally results in bacterial cell death.[1]
Polymyxin B was approved for medical use in the United States in 1964.[1] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[1] In the United States it costs about US$17 per day.[8] In Europe it is only approved to be applied to the skin as of 2015.[9] It is derived from the bacterium Bacillus polymyxa.[4]
Medical uses
.png.webp)
Spectrum of susceptibility
Polymyxin B has been used to treat urinary tract infections and meningitis caused by Pseudomonas aeruginosa and Haemophilus influenzae, respectively. The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: ≥0.8 μg/ml
- Pseudomonas aeruginosa: 0.25 μg/ml – 1 μg/ml[10]
Endotoxin adsorption
An endotoxin removal cartridge (Toraymyxin) is a blood purification medical device and it uses polymyxin B as immobilized adsorbent.[11]
Dosage
The defined daily dose is 150 mg by injection[2] and 3 million units by mouth.[3]
Mechanism of action
- Alters bacterial outer membrane permeability by binding to a negatively charged site in the lipopolysaccharide layer, which has an electrostatic attraction for the positively charged amino groups in the cyclic peptide portion [12] (this site normally is a binding site for calcium and magnesium counter ions); the result is a destabilized outer membrane
- Fatty acid portion dissolves in hydrophobic region of cytoplasmic membrane and disrupts membrane integrity
- Leakage of cellular molecules, inhibition of cellular respiration
- Binds and inactivates endotoxin[13]
- Relative absence of selective toxicity: nonspecific for cell membranes of any type, highly toxic.
Mixture composition
Polymyxin B is composed of polymyxins B1, B1-I, B2, B3, and B6. Polymyxins B1 and B2 are considered major components. These related components are structurally identical with the exception of a variable fatty acid group on each fraction. Results from in vitro studies have shown marginal differences in MIC data when comparing the fractions.[14]
Research application
Polymyxin B is also used to induce envelope stress in order to study the organisms response to such stress. Polymyxin envelope stress assays such as this have been used for the study of small RNA (sRNA) responses in Salmonella enterica.[15]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 "Polymyxin B Sulfate topical Monograph for Professionals". Drugs.com. Archived from the original on 11 November 2019. Retrieved 11 November 2019.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 24 September 2020. Retrieved 10 September 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 29 December 2019. Retrieved 10 September 2020.
- 1 2 Bennett, John E.; Dolin, Raphael; Blaser, Martin J.; Mandell, Gerald L. (2009). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases E-Book. Elsevier Health Sciences. p. 469. ISBN 9781437720600. Archived from the original on 2021-08-28. Retrieved 2019-11-11.
- ↑ "DailyMed - neomycin, bacitracin, polymyxin b ointment". dailymed.nlm.nih.gov. Archived from the original on 11 July 2021. Retrieved 19 April 2019.
- ↑ Woo, Teri Moser; Robinson, Marylou V. (2015). Pharmacotherapeutics For Advanced Practice Nurse Prescribers. F.A. Davis. p. 651. ISBN 9780803645813. Archived from the original on 2019-04-19. Retrieved 2019-11-11.
- ↑ Organization, World Health (2019). "World Health Organization model list of essential medicines: 21st list 2019". hdl:10665/325771.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Polymyxin b Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 11 November 2019. Retrieved 11 November 2019.
Typical dose is about 20,000 units per kg * 70 = 1.4 million. So three vials per day.
- ↑ "Polymyxin-based products" (PDF). EMA. Archived (PDF) from the original on 11 November 2019. Retrieved 11 November 2019.
- ↑ "Polymyxin B sulfate : Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). Toku-e.com. Archived from the original on 2018-08-05. Retrieved 2017-04-02.
- ↑ Shoji H. (February 2003). "Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin)". Therapeutic Apheresis and Dialysis. 7 (1): 108–114. doi:10.1046/j.1526-0968.2003.00005.x. PMID 12921125.
- ↑ Khondker A, Dhaliwal AK, Saem S, Mahmood A, Fradin C, Moran-Mirabal J, Rheinstadter MC (February 2019). "Membrane charge and lipid packing determine polymyxin-induced membrane damage". Communications Biology. 2: 69. doi:10.1038/s42003-019-0297-6. PMC 6379423. PMID 30793045.
- ↑ Cardoso LS, Araujo MI, Góes AM, Pacífico LG, Oliveira RR, Oliveira SC (January 2007). "Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis". Microbial Cell Factories. 6: 1. doi:10.1186/1475-2859-6-1. PMC 1766364. PMID 17201926.
- ↑ Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J (2001). "Isolation and structural characterization of polymyxin B components". Journal of Chromatography A. 912 (2): 369–373. doi:10.1016/S0021-9673(01)00585-4. PMID 11330807.
- ↑ Hébrard M, Kröger C, Srikumar S, Colgan A, Händler K, Hinton JC (April 2012). "sRNAs and the virulence of Salmonella enterica serovar Typhimurium". RNA Biology. 9 (4): 437–45. doi:10.4161/rna.20480. PMC 3384567. PMID 22546935.
External links
| Identifiers: |
|---|
- "Polymyxin B". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2019-11-11. Retrieved 2019-11-11.