Barbital
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682221 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 30.3 (± 3.2) hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.301 |
| Chemical and physical data | |
| Formula | C8H12N2O3 |
| Molar mass | 184.195 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Barbital (or barbitone), marketed under the brand names Veronal for the pure acid and Medinal for the sodium salt, was the first commercially available barbiturate. It was used as a sleeping aid (hypnotic) from 1903 until the mid-1950s. The chemical names for barbital are diethylmalonyl urea or diethylbarbituric acid; hence, the sodium salt (known as medinal, a genericised trademark in the United Kingdom) is known also as sodium diethylbarbiturate.
Synthesis
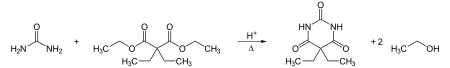
Barbital, then called "Veronal", was first synthesized in 1902 by German chemists Emil Fischer and Joseph von Mering, who published their discovery in 1903.[1] Barbital was prepared by condensing diethylmalonic ester with urea in the presence of sodium ethoxide, or by adding at least two molar equivalents of ethyl iodide to the silver salt of malonylurea (barbituric acid) or possibly to a basic solution of the acid. The result was an odorless, slightly bitter, white crystalline powder.[2]
Its introduction followed the investigations of Fischer and von Mering on the pharmacological properties of certain open and closed acylureas (then called ureides). Led by the impression that hypnotic action appears to be largely dependent on the presence of ethyl groups, they prepared diethylacetyl urea, diethylmalonyl urea (i.e., Barbital itself), and dipropylmalonyl urea. All three were found to be hypnotics: the first was about equal in power to the already-known sulphonal (now sulfonmethane), whilst the third was four times as powerful, but its use was attended by prolonged after-effects. Veronal was found to be midway.[2]
Barbital can also be synthesized in a condensation reaction from urea and diethyl-2,2-diethylmalonate, a diethyl malonate derivative:
Marketing

Barbital was marketed in 1904 by the Bayer company as “Veronal”. A soluble salt of barbital was marketed by the Schering company as “Medinal.” It was dispensed for “insomnia induced by nervous excitability”.[3] It was provided in either crystal form or in cachets (capsules). The therapeutic dose was ten to fifteen grains (0.6-1 grams). 3.5 to 4.4 grams (55 to 68 grains) is the deadly dose but sleep has also been prolonged up to ten days with recovery.
Pharmacology
Barbital was considered to be a great improvement over the existing hypnotics. Its taste was slightly bitter, but better than the strong, unpleasant taste of the commonly used bromides. It had few side effects, and its therapeutic dose was far below the toxic dose. However, prolonged usage resulted in tolerance to the drug, requiring higher doses to reach the desired effect. "I'm literally saturated with it," the Russian tsarina Alexandra Feodorovna confessed to a friend.[4] Fatal overdoses of this slow-acting hypnotic were not uncommon. Pioneering aviator Arthur Whitten Brown (of "Alcock and Brown" fame) died of an accidental overdose.
pH buffer
Solutions of sodium barbital have also been used as pH buffers for biological research, e.g., in immunoelectrophoresis or in fixative solutions.[5][6] As barbital is a controlled substance, barbital-based buffers have largely been replaced by other substances.[7]
Suicide
Japanese writer Ryūnosuke Akutagawa deliberately overdosed on the drug in 1927, as did Un Chien Andalou actor Pierre Batcheff in 1932, Austrian writer Stefan Zweig in 1942 and Greek musician Attik in 1944. During The Holocaust, many Jewish residents of Berlin, Dresden, Wiesbaden and other German cities used Veronal to commit suicide to avoid deportation to concentration camps by the Nazi[8] Regime.[9] Alfred Kerr German theatre critic and essayist on a trip to Germany after WWII, suffered a stroke, and decided to end his own life via an overdose of Veronal, procured for him by his wife[10]
In the D. H. Lawrence story, The Lovely Lady, the titular character dies from a self-administered overdose.[11]
Barbital, under the name of "Veronal", has been used as a plot device in the author Agatha Christie's murder mysteries.[12]
References
- ↑ Fischer E, von Mering J (1903). "Ueber eine neue Klasse von Schlafmitteln" [About a new class of sleeping pills]. Therapie der Gegenwart (in German). 44: 97–101.
- 1 2 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Veronal". Encyclopædia Britannica. Vol. 27 (11th ed.). Cambridge University Press. p. 1037.
- ↑ Finley E (1919). "Veronal". The American Materia Medica, Therapeutics and Pharmacognosy. p. 115. Retrieved 25 July 2015.
- ↑ Dehn L (1922). The Real Tsaritsa. Boston: Little Brown. p. 138.
- ↑ Kuhlmann WD (10 September 2006). "Buffer Solutions" (PDF). Archived from the original (PDF) on 9 November 2016. Retrieved 28 July 2014.
- ↑ Ruzin SE (1999). Plant Microtechnique and Microscopy. Oxford University Press. Archived from the original on 3 June 2019. Retrieved 28 July 2014.
- ↑ Monthony JF, Wallace EG, Allen DM (October 1978). "A non-barbital buffer for immunoelectrophoresis and zone electrophoresis in agarose gels". Clinical Chemistry. 24 (10): 1825–7. doi:10.1093/clinchem/24.10.1825. PMID 568042.
- ↑ I Will Bear Witness by Victor Klemperer (Author), Martin Chalmers (Translator) 1998
- ↑ Cargas, Harry J. (1999). Problems Unique to the Holocaust. Univ Pr of Kentucky. p. 44. ISBN 9780813121017.
- ↑ "Im Interview: Judith Kerr - Wir waren eine Insel - Kultur - sueddeutsche.de". 2008-06-10. Archived from the original on 2008-06-10. Retrieved 2021-07-08.
- ↑ Lawrence, D. H. (David Herbert) (1988). The virgin and the gypsy : and other stories. Internet Archive. London : Marshall Cavendish. ISBN 978-0-86307-694-7.
- ↑ "A quote from The Murder of Roger Ackroyd". www.goodreads.com. Retrieved 2021-10-22.
Further reading
- Dombrowski SM, Krishnan R, Witte M, Maitra S, Diesing C, Waters LC, Ganguly R (October 1998). "Constitutive and barbital-induced expression of the Cyp6a2 allele of a high producer strain of CYP6A2 in the genetic background of a low producer strain". Gene. 221 (1): 69–77. doi:10.1016/s0378-1119(98)00436-3. PMID 9852951.
- Norena Shopland T he Veronal Mystery (Wordcatcher Publishing) 2020