3CLpro-1
 | |
| Clinical data | |
|---|---|
| Trade names | 3CLpro-1 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
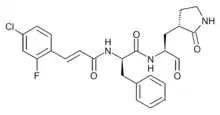
| Formula | C25H25ClFN3O4 |
| Molar mass | 640.8 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3CLpro-1 is an antiviral drug related to rupintrivir which acts as a 3CL protease inhibitor and was originally developed for the treatment of human enterovirus 71. It is one of the most potent of a large series of compounds developed as inhibitors of the viral enzyme 3CL protease, with an in vitro IC50 of 200 nM. It also shows activity against coronavirus diseases such as SARS and MERS, and is under investigation as a potential treatment agent for the viral disease COVID-19.[1][2][3][4][5][6][7]
See also
- Carmofur
- Ebselen
- GC376
- GRL-0617
- Rupintrivir
- Theaflavin digallate
References
- ↑ Kuo CJ, Shie JJ, Fang JM, Yen GR, Hsu JT, Liu HG, et al. (August 2008). "Design, synthesis, and evaluation of 3C protease inhibitors as anti-enterovirus 71 agents". Bioorganic & Medicinal Chemistry. 16 (15): 7388–98. doi:10.1016/j.bmc.2008.06.015. PMC 7125518. PMID 18583140.
- ↑ Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R, Nunneley JW, et al. (April 2015). "Protease inhibitors targeting coronavirus and filovirus entry". Antiviral Research. 116: 76–84. doi:10.1016/j.antiviral.2015.01.011. PMC 4774534. PMID 25666761.
- ↑ Kumar V, Shin JS, Shie JJ, Ku KB, Kim C, Go YY, et al. (May 2017). "Identification and Evaluation of Potent Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 3CL Pro Inhibitors". Antiviral Research. 141: 101–106. doi:10.1016/j.antiviral.2017.02.007. PMC 7113684. PMID 28216367.
- ↑ Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. (2020). "Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases". ACS Central Science. 6 (3): 315–331. doi:10.1021/acscentsci.0c00272. PMC 7094090. PMID 32226821.
- ↑ Morse JS, Lalonde T, Xu S, Liu WR (March 2020). "Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV". ChemBioChem. 21 (5): 730–738. doi:10.1002/cbic.202000047. PMC 7162020. PMID 32022370.
- ↑ Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, et al. (February 2020). "α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment". Journal of Medicinal Chemistry. 63 (9): 4562–4578. doi:10.1021/acs.jmedchem.9b01828. PMC 7098070. PMID 32045235.
- ↑ Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, et al. (March 2020). "Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors". Science. 368 (6489): 409–412. doi:10.1126/science.abb3405. PMC 7164518. PMID 32198291.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.